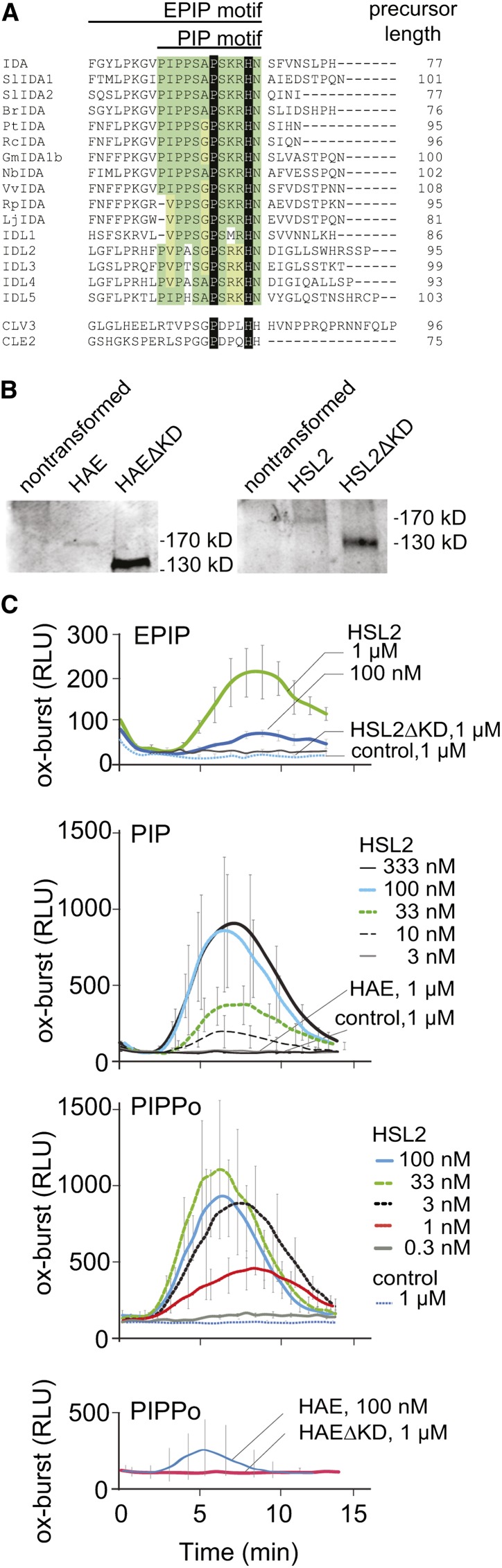
Figure 1.
Ox-Burst by the IDA-HAE/HSL2 Signaling Module.
(A) Alignment of the C-terminal region of IDA, IDL1-5 from Arabidopsis, and IDA orthologs from other species, CLV3 and CLE2. Sequence similarities within the PIP domain of the IDA peptides are highlighted in green. The invariant proline, hydroxyprolinated in CLV3, CLE2, and presumably also IDA, and the His conserved in all these signal peptides are indicated in black.
(B) Protein gel blot of N. benthamiana leaves expressing Halo-tagged HAE and HSL2 as full-length constructs or as kinase truncated versions (ΔKD), respectively. Blots developed with α-Halo antibody stain single bands that migrate with molecular weights that are higher than those anticipated by the polypeptide chains alone. This is likely due to glycosylation of the receptors, as previously reported for HAE (Jinn et al., 2000).
(C) IDA triggers the production of ROS through HSL2 in N. benthamiana. Oxidative burst was measured in leaf pieces of untransformed N. benthamiana (control) or leaves transiently expressing the HSL2 receptor or HSL2ΔKD, HAE, or HAEΔKD as indicated. Leaf pieces were exposed to various concentration of the EPIP peptide of IDA (top panel), the PIP peptide (second panel from top), or the peptide PIPPo, PIP with hydroxylation of the conserved Pro at position 7 (bottom two panels). Ox-burst by the luminol-based assay was monitored over time as relative light units (RLU). Error bars indicate sd of n = 3 replicates.