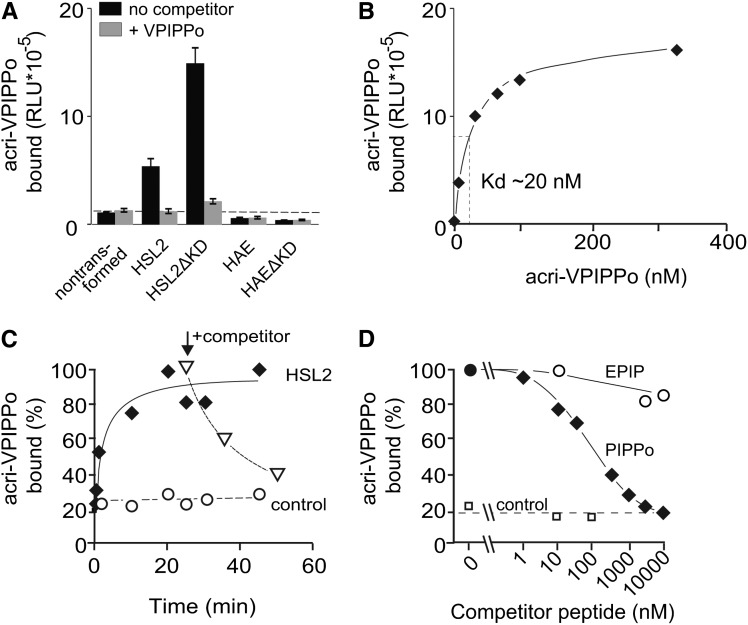
Figure 3.
Binding of the IDA-Derived Peptide Acri-PIPPo to HSL2.
(A) Modified IDA peptides bind directly to the ectodomain of HSL2. Leaf material from N. benthamiana expressing HSL2, HAE, or the truncated version HSL2ΔKD or HAEΔKD lacking the kinase domain was incubated with 10 nM acri-PIPPo peptide in the presence or absence of 10 μM unlabeled VPIPPo as a competitor. Controls show binding to leaf material from nontransformed N. benthamiana. Bars and error bars show averages and standard deviations of n = 3 replicates. RLU, relative light units.
(B) Saturation of acri-PIPPo binding to the HSL2 receptor. Specific binding of acridinium-tagged VPIPPo was determined by subtracting binding observed with untransformed leaf material at the different concentrations from the values measured for the HSL2 expressing leaf material.
(C) Binding kinetics and reversibility of binding to the HSL2 receptor. Plant material from HSL2 expressing or nonexpressing (control) leaf samples were incubated with 10 nM acri-PIPPo. Reversibility of binding was tested by adding 10 μM of VPIPPo (indicated with an arrow) at 25 min. Binding is expressed as percentage of maximal binding in material expressing HSL2.
(D) HSL2-Halo expressing leaf material incubated with acri-PIPPo and increasing concentrations of EPIP and PIPPo as competitors. Leaf tissue not expressing the receptor was incubated with the acri-PIPPo peptide and different concentrations of PIPPo as a control to determine background signal. The signal detected from samples without competitor was defined as 100% binding.