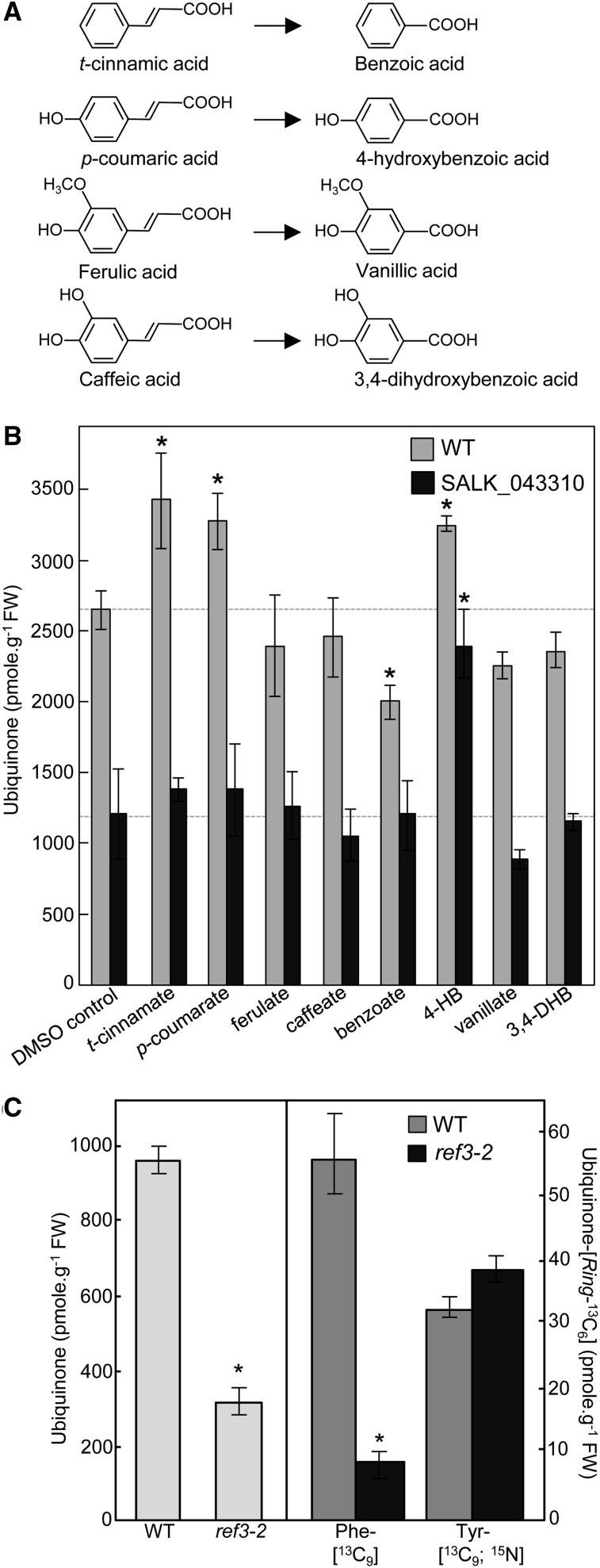
Figure 4.
t-Cinnamate, p-Coumarate, and 4-Hydroxybenzoate Serve as Precursors of Ubiquinone in Arabidopsis.
(A) Structures of t-cinnamic, p-coumaric, ferulic and caffeic acids, and their corresponding shortened aliphatic side-chain versions.
(B) Total ubiquinone (reduced and oxidized) levels in axenically grown wild-type and SALK_043310 Arabidopsis plants fed for 24 h with 200 μM t-cinnamate, p-coumarate, ferulate, caffeate, benzoate, 4-hydroxybenzoate (4-HB), vanillate, or 3,4-dihydroxybenzoate (3,4-DHB). Data are means of four biological replicates ± se. Asterisk annotations indicate significant difference from the corresponding DMSO control as determined by Fisher’s test (P < α = 0.05) from an analysis of variance. FW, fresh weight.
(C) Left panel, total ubiquinone levels in the leaves of the wild type and cinnamate-4 hydroxylase mutant (ref3-2) Arabidopsis plants. Right panel, total ubiquinone-[Ring-13C6] levels in axenically grown wild-type and ref3-2 Arabidopsis plants fed with 250 μM doses of Phe-[13C9] or Tyr-[13C9;15N] for 3 h. Data are means of four biological replicates ± se. Asterisk annotations indicate significant difference from the wild type as determined by Fisher’s test (P < α = 0.05) from an analysis of variance.