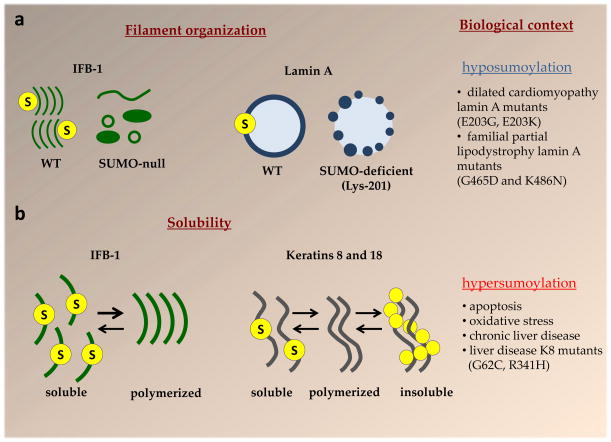
Figure 3. IF protein sumoylation regulates filament organization and solubility properties.
a. Sumoylation regulates the organization of IFs. Sumoylation is important for the proper filament organization of IFB-1, a C. elegans cytoplasmic IF that forms epidermal attachment structures12. In SUMO-null worms IFB-1 filaments appear as a heterogeneous population of different size aggregates, long filaments and ring-like structures, and the occurrence of large aggregates increases with age. 12 Similarly, sumoylation of human lamin A in the highly-conserved rod domain at Lys-201 is important for its proper nuclear localization14. The sumoylation deficient lamin A K201R forms abnormal puncta inside the nuclei or at the nuclear periphery14. Laminopathy-associated lamin A mutants, as exemplified by those listed in the figure, are hyposumoylated and exhibit abnormal organization in comparison to normal lamin A14,96. b. Sumoylation regulates the solubility of IFs. Sumoylation sequesters a fraction of IFB-1 into a soluble cytoplasmic pool and facilitates the exchange between the soluble and polymerized IFB-1 pool12. Sumoylation of wild-type polymerized human K8 and K18 (depicted by the yellow filaments in the middle) is not readily detectable under basal but is highly upregulated upon the re-organization of these IFs in response to apoptosis, stress, disease, or in the context of K8 variants that predispose to liver disease13. Monosumoylation promotes K8 solubility in vitro, similar to IFB-1, whereas pathologic hypersumoylation is associated with diminished K8 solubility and formation of high molecular mass complexes13.