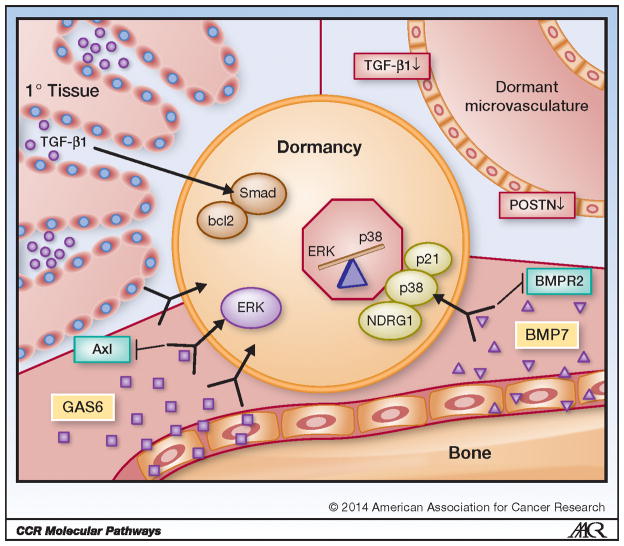
Figure 1. Niche Mediated Tumor Cell Dormancy.
Tumor cells become and stay dormant via signaling from the surrounding tissue (niche). High levels of transforming growth factor beta-1 (TGF-β1) in the proximal region of primary tissue cause activation of Smad and upregulation of the apoptosis suppressor gene bcl2, which in turn induces dormancy in resident tumor cells. Tumor cells have also been shown to enter dormancy within dormant microvasculature, which is believed to occur because of characteristically low levels of TGF-β1 and periostin (POSTN). Osteoblast derived growth arrest specific 6 (GAS6), and stromal cell derived bone morphogenetic protein 7 (BMP7) in the bone marrow also induces tumor cell dormancy. GAS6 is recognized by tyrosine kinase receptor Axl, which activates the extracellular signal-regulated kinase (ERK) mitogen-activated protein kinase (MAPK) pathway. BMP7 is received by BMP receptor 2 (BMPR2), which causes activation of cell cycle inhibitor p21, upregulation of N-myc downstream-regulated gene 1 (NDRG1) and p38 MAPK phosphorylation. Strong evidence suggests tumor cells respond to external stimuli by shifting the balance of the ERK MAPK and p38 MAPK pathways: in dormant tumor cells the p38 MAPK pathway is generally more activated than ERK MAPK.