SUMMARY
CD4 T cell activation leads to rapid proliferation and differentiation into effector (Teff) or regulatory (Treg) cells that mediate or control immunity. While Teff and Treg prefer distinct glycolytic or oxidative metabolic programs in vitro, requirements and mechanisms that control T cell glucose uptake and metabolism in vivo are poorly understood. Despite expression of multiple glucose transporters, Glut1-deficiency selectively impaired metabolism and function of thymocytes and Teff. Resting T cells were normal until activated, when Glut1-deficiency prevented increased glucose uptake and glycolysis, growth, proliferation, and decreased cell survival and Teff differentiation. Importantly, Glut1-deficiency decreased Teff expansion and ability to induce inflammatory disease in vivo. Treg, in contrast, were enriched in vivo and appeared functionally unaffected by Glut1-deficiency and able to suppress Teff irrespective of Glut1 expression. These data show a selective in vivo requirement for Glut1 in metabolic reprogramming of CD4 T cell activation and Teff expansion and survival.
INTRODUCTION
T cell activation initiates a transition from quiescence to rapid cell growth, proliferation, and differentiation into functional subsets to drive or suppress the immune response (Zhu et al., 2010). Effector CD4 T cells (Teff; including Th1, Th2, and Th17) promote immunity and are enriched in inflammatory diseases. Regulatory CD4 T cells (Treg), in contrast, suppress immunity and are decreased in number or function in these settings (Zhu et al., 2010). Importantly, the transition from quiescence to rapid growth and proliferation increases energetic and biosynthetic demands. Activated T cells thus upregulate nutrient uptake and metabolic rates (MacIver et al., 2013), resulting in a significant elevation of glucose and amino acid transport (Frauwirth et al., 2002; Sinclair et al., 2013; Wang et al., 2011) that may provide new directions to modulate immunity. T cell metabolism has been shown in distinct settings to require lipid synthesis (Kidani et al., 2013) or oxidation (Byersdorfer et al., 2013; Gatza et al., 2011), mitochondrial reactive oxygen species (Sena et al., 2013), and amino acid uptake (Sinclair et al., 2013). However, the in vivo mechanism and roles of increased glucose uptake and metabolism in T cell-mediated inflammatory diseases remain uncertain.
It is now evident that metabolic reprogramming is shaped to support specific cell functions (MacIver et al., 2013). In vitro generated Teff strongly induce glycolysis and decrease lipid oxidation (Michalek et al., 2011a; Shi et al., 2011; Wang et al., 2011). In contrast, in vitro induced Treg and memory CD8 T cells utilize lipid oxidation as a primary metabolic pathway (Michalek et al., 2011a; Pearce et al., 2009; Shi et al., 2011). These metabolic programs provide distinct metabolites (MacIver et al., 2013), signaling through the mTORC1 pathway (Sinclair et al., 2013), and cytokine production (Cham and Gajewski, 2005; Chang et al., 2013). Importantly, induced Teff and Treg may be differentially sensitive to glycolytic inhibition, as in vitro glucose limitation or 2-deoxyglucose (2DG) treatment suppressed Th17 but not Treg cells (Michalek et al., 2011a; Shi et al., 2011). Because these pharmacologic approaches result in broad non-specific effects that impact all cells, mechanistic insight has been limited. A genetic approach is needed to establish the cell-intrinsic roles of glucose metabolism in T cell activation and regulation of inflammation.
Glucose uptake provides a key metabolic control point through the Glut family of facilitative glucose transporters. The fourteen Glut family members are differentially regulated and possess distinct substrates and biological properties (Thorens and Mueckler, 2010). The array of Glut transporters utilized by T cells in activation and differentiation has not yet been defined. In vitro stimulated murine and human T cells express high levels of Glut1 (Slc2a1) (Frauwirth et al., 2002; Jacobs et al., 2008) and Teff cells maintain higher levels of Glut1 than Treg (Michalek et al., 2011a). Glut1 can promote Teff, as transgenic Glut1 overexpression selectively increased Teff frequency and led to inflammatory disease (Jacobs et al., 2008; Michalek et al., 2011a).
Here, we examine the Glut transporter family to directly test the role and mechanisms that control T cell glucose uptake and metabolism in activation and in inflammatory disease. Although T cells expressed four distinct glucose transporter isoforms, genetic targeting showed a selective role for Glut1 in proliferative thymocytes and Teff cells. While resting murine and human T cells were independent of Glut1, Teff required Glut1 for efficient expansion and specification in vivo. In contrast, both natural and induced Treg were independent of Glut1. As a result, Glut1-deficient Teff were unable to effectively induce either Graft-vs-Host Disease (GvHD) or colitis, while Treg appeared suppressive independent of Glut1. Thus, Glut1 has a selective cell-intrinsic function in T cell metabolic reprogramming to drive glycolysis of Teff for growth, expansion, and inflammatory disease.
RESULTS
T cells express a subset of dynamically regulated Glut family transporters
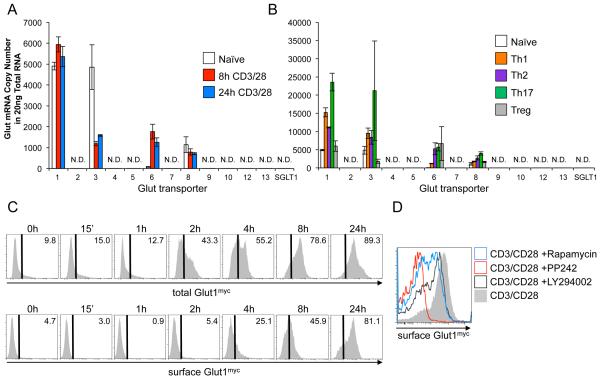
The mechanism of glucose uptake and role of Glut family glucose transporters in activation-induced glucose uptake in T cells has not been directly established. The absolute expression of each Glut family member was, therefore, examined. mRNA transcript copy number was quantified in resting and activated murine T cells (Fig. 1A). Of the thirteen glucose transporter family members measured, only Glut 1, 3, 6, and 8 were detected. Slc2a1 (Glut1) and Slc2a3 (Glut3) mRNA were equally expressed in resting CD4 T cells. Following activation, Slc2a1 (Glut1) was induced or sustained, while Slc2a3 (Glut3) mRNA became less prominent. Slc2a6 (Glut6) was also induced with activation, but remained at lower copy number than Slc2a1 (Glut1). Glut family member expression was also measured in induced Th1, Th2, Th17, and Treg (Fig. 1B). Again, Gluts 1, 3, 6, and 8 were the only detectable Glut transporters, and while each T cell subset had a distinct transporter profile, Glut1 was found in the highest copy number in each case. Of note, differentiated cells expressed Glut3 more similarly to Glut1.
Figure 1. Glut1 is selectively and rapidly increased in activated murine T cell activation.
(A, B) Glut family mRNA copy number in (A) naïve and CD3/CD28-stimulated CD4 murine T cells, and (B) in vitro polarized CD4 T cell subsets. N.D.: not detected. (C, D) Glut1myc expression in CD3/CD28 stimulated CD4 Glut1myc T cells (C) over time and (D) with inhibitors or vehicle control. Mean ± SD from 3 or more independent experiments are shown.
In addition to mRNA levels, the trafficking of Glut1 to the cell surface is also highly regulated (McCracken and Edinger, 2013; Wieman et al., 2007). Glut1 protein levels and trafficking were therefore examined during T cell activation. Because available antibodies react poorly with the extracellular domains of Glut1, Glut1myc knock-in mice were used to measure cell surface Glut1 in T cell development and activation. In this model a tandem-Myc tag was knocked into exon 3 of Slc2a1, which encodes the large exofacial loop of Glut1, enabling sensitive and specific flow cytometric measurement of endogenous cell surface Glut1 using antibodies against the Myc epitope (Michalek et al., 2011a).
Increased total intracellular expression of Glut1myc was detected within two hours of CD3 and CD28 stimulation. Cell surface levels of Glut1myc increased more slowly, but elevated Glut1myc surface expression were measurable within four hours of stimulation and maximal after 24 hours (Fig. 1C). Consistent with previous data implicating the PI3K-Akt-mTORC1 pathway in the regulation of Glut1 expression and trafficking (Frauwirth et al., 2002; Wieman et al., 2007), inhibitors of PI3K (LY294002), PI3K/mTOR kinase (PP242), or mTORC1 (rapamycin) suppressed the induction of cell surface Glut1 (Fig. 1D). Glut1 upregulation and cell surface trafficking is, therefore, an early event in T cell activation mediated in part through PI3K-Akt-mTORC1 signaling.
Glut1 supports thymic development but is not required for resting peripheral T cell survival
Based on the dynamic expression of Glut1, the specific role of this transporter was tested in T cell development and activation. Glut1fl/fl mice (Young et al., 2011) were crossed with LckCre transgenic mice to specifically delete Glut1 in early DN thymocytes. Total thymocyte numbers were reduced 60-70% in LckCreGlut1fl/fl mice compared to control animals (Fig. S1A). This reflected a sharp loss of CD4 CD8 double positive (DP) and CD4 single positive (SP) cells, while double negative (DN) cell numbers were unchanged and represented an increased fraction of thymocytes (Figs. S1B, C). Within the DN population, LckCreGlut1fl/fl mice had normal numbers of DN1, DN2, and DN3, and a trend towards reduced DN4 cells (Fig. S1C). Glut1 therefore contributes to, but is not required for, thymocyte differentiation. Importantly, the DN3-DN4 stages at which Glut1-deficient cells began to show defects corresponding to the highest surface Glut1 expression (Fig. S1D) and most proliferative phase of thymopoiesis (Shortman et al., 1990).
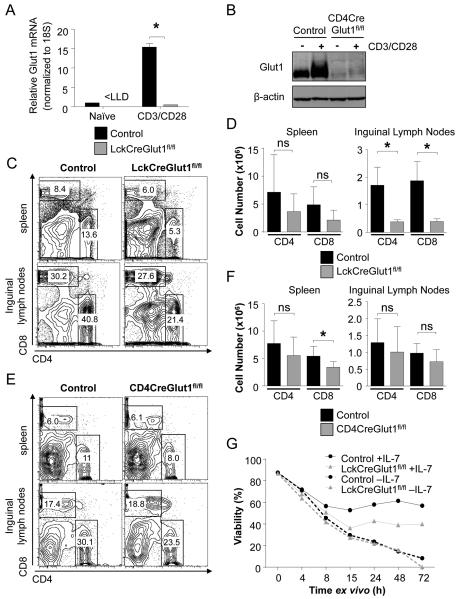
Persistent SP thymocytes and peripheral T cells may have failed to delete or may not require Glut1 for development or survival. To bypass potential developmental defects, Glut1fl/fl mice were crossed to CD4Cre transgenic mice, which delete loxP flanked genes in the DP to SP transition following the proliferative DN3-DN4 stage in thymopoiesis. Thymocyte numbers and phenotype were normal in CD4CreGlut1fl/fl mice (Figs. S2A, B). Importantly, peripheral T cells present in both LckCreGlut1fl/fl and CD4CreGlut1fl/fl mice had efficiently deleted Glut1 as measured by mRNA and protein (Figs. 2A, B). The numbers of lymph node T cells in LckCreGlut1fl/fl mice, however, were reduced (Figs. 2C,D). In contrast, peripheral T cell numbers in CD4CreGlut1fl/fl mice trended towards only a modest reduction, with the only significant difference being fewer Glut1-deficient CD8 T cells in the spleen (Figs. 2E, F).
Figure 2. Glut1 is not required for survival of quiescent peripheral T cells.
(A, B) Glut1fl/fl and LckCreGlut1fl/fl or CD4CreGlut1fl/fl T cells were rested in IL-7 (naive) or CD3/CD28-stimulated for 16h and Glut1 levels measured by (A) qrtPCR or (B) immunoblot. (C-F) Flow cytometry (C, E) and cell numbers (D, F) are shown for CD4 and CD8 cells from spleen and inguinal lymph nodes of control and (C and D) LckCreGlut1fl/fl or (E and F) CD4CreGlut1fl/fl mice. (G) Viability of isolated control (Glut1fl/+) and LckCreGlut1fl/fl T cells over time in culture ± IL-7. Mean cell count ± SD or representative data from (C, D) 7 and (E, F) 5 animals and (A, B, G) 2 or more independent experiments are shown.
The dependence of peripheral T cells on Glut1 for survival was directly tested by measuring cell death in cultures of resting control or LckCreGlut1fl/fl T cells with or without the addition of IL-7 (Fig. 2G). IL-7 plays a key role to determine the survival and basal rates of glucose metabolism of resting T cells (Carrette and Surh, 2012; Jacobs et al., 2010; Rathmell et al., 2001). Both control and Glut1-deficient T cells underwent apoptosis at the same rate in the absence of IL-7. Treatment with IL-7 protected resting control T cells from death. Glut1-deficient T cells had moderately lower survival than control cells when cultured with IL-7, but nonetheless were also largely protected from apoptosis. Resting peripheral T cells thus do not require Glut1 for survival.
Glut1 is required for growth and proliferation of mature T cells
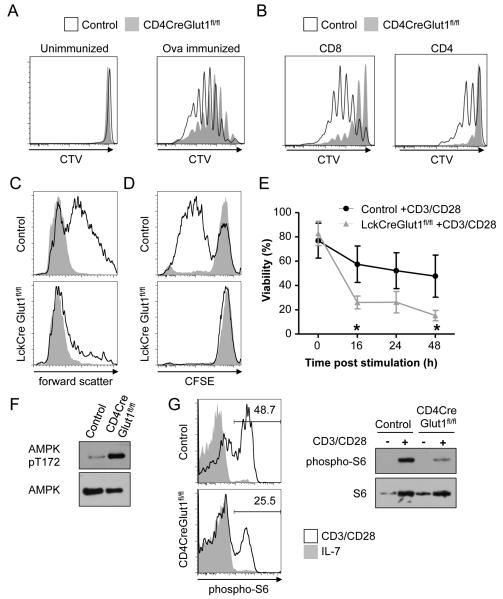
The decreased numbers of LckCreGlut1fl/fl thymocytes after the proliferative DN3-DN4 phase and apparent Glut1-independence of resting T cell survival suggested that Glut1 is selectively required to support proliferation. To test the dependence of peripheral T cells on Glut1 for activation-induced proliferation, Ovalbumin (Ova)-specific OT-II T cell receptor (TCR) transgenic control or CD4CreGlut1fl/fl CD4 T cells were labeled with the proliferation dye CellTrace Violet and adoptively transferred into Thy1.1 hosts that were then immunized with Ova. Importantly, Glut1-deficient CD4 T cells had reduced proliferation (Fig. 3A). We also tested the ability of Glut1-deficient T cells to undergo homeostatic proliferation after adoptive transfer into irradiated and lymphopenic recipients. Consistent with a need for Glut1 to allow proliferation in vivo, Glut1-deficient CD8 and CD4 T cells underwent only limited homeostatic proliferation (Fig. 3B).
Figure 3. Glut1 is necessary to support activation-induced growth, proliferation and survival.
(A, B) Proliferation of CellTrace Violet (CTV) labeled control (Glut1fl/fl) and CD4CreGlut1fl/fl (A) OT-II transgenic T cells on day 3 after adoptive transfer into intact recipients ± immunization with Ovalbumin or (B) T cells 6 days after adoptive transfer into irradiated recipients for homeostatic proliferation. (C) Control (Glut1fl/fl) and LckCreGlut1fl/fl T cells were rested in IL-7 or CD3/CD28-stimulated and cell size (forward scatter) of viable cells was determined by flow cytometry after 24h. (D, E) Control (Glut1fl/+) and LckCreGlut1fl/fl T cells were CFSE-labeled and either rested in IL-7 or CD3/CD28-stimulated and examined by flow cytometry for (D) proliferation at 72 hours or (E) viability over time. (F) Control (Glut1fl/fl) and CD4CreGlut1fl/fl T cells were CD3/CD28-stimulated 16h and analyzed by immunoblot. (G) Control (Glut1fl/fl) and CD4CreGlut1fl/fl T cells were rested in IL-7 or CD3/CD28-stimulated for 10h and analyzed by intracellular flow cytometry and immunoblot. Data are representative of n=3 mice/group (A, B) a minimum of (F, G) 2 or (C-E) 3 experiments. (E) Shows mean ± SD of 3 independent experiments.
T cells were stimulated in vitro to examine mechanisms that suppressed proliferation and accumulation of Glut1-deficient T cells in vivo. Importantly, in vitro stimulated Glut1-deficient T cells grew very poorly, as evidenced by a failure to increase forward light scatter by flow cytometry (Fig. 3C). Further, activation sufficient to drive the proliferation of control T cells failed to induce proliferation (Fig. 3D) and instead led to rapid induction of cell death of many LckCreGlut1fl/fl T cells (Fig. 3E). Viable Glut1-deficient T cells had increased levels of phospho-AMPK after activation (Fig. 3F), suggesting these T cells are under metabolic stress. Further these cells failed to sustain activated mTORC1 signaling, as assessed by phosphorylation of the downstream p70 S6 kinase target, small ribosomal subunit S6 (Fig. 3G). The mTOR pathway can broadly regulate nutrient uptake (McCracken and Edinger, 2013), and the transferrin receptor (CD71) and the 4F2hc amino acid transporter (CD98) were not efficiently induced in activated Glut1-deficient T cells (Fig. S3A). Most signaling pathways, however, were unchanged, as cMyc was induced and phospho-Akt and -ERK were equivalent or only modestly reduced in activated Glut1-deficient T cells (Fig. S3B). Activation markers were also induced normally (Fig. S3C).
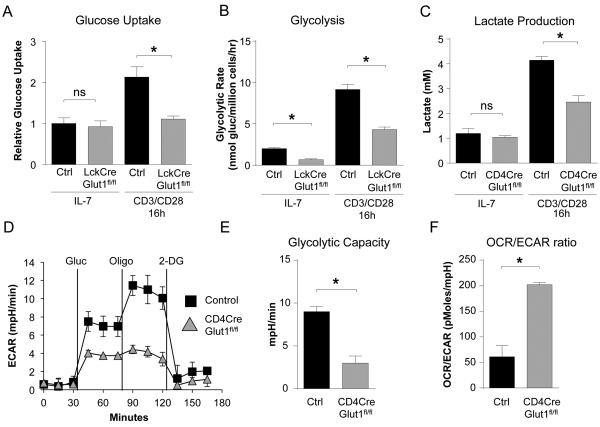
Glucose uptake and glycolysis were measured in resting and activated T cells to assess the metabolic role of Glut1. Consistent with a selective role in activation, resting IL-7 treated peripheral T cells did not rely on Glut1 and had similar rates of glucose uptake, glycolysis, and lactate production regardless of Glut1 expression (Fig. 4A, B, C). However, while control T cells rapidly increased glucose metabolism after activation, stimulated LckCreGlut1fl/fl T cells maintained only a basal rate of glucose uptake and greatly reduced glycolytic rate (Fig. 4A, B). Likewise, stimulated CD4CreGlut1fl/fl T cells failed to increase lactate production when measured directly or by extracellular acidification rate (ECAR) (Fig. 4C, D). As a consequence, activated Glut1-deficient T cells had reduced glycolytic capacity (Fig. 4E) and an elevated ratio of oxygen consumption rate (OCR) to ECAR (Fig. 4F). Glut1 is, therefore, essential for rapid metabolic reprogramming to aerobic glycolysis for maximal growth, survival, and proliferation of in vitro stimulated T cells.
Figure 4. Glut1 is required for activation-induced metabolic reprogramming.
(A, B) Control (Glut1fl/fl) and LckCreGlut1fl/fl T cells were rested in IL-7 or CD3/CD28-stimulated and (A) glucose uptake or (B) glycolytic rate was measured after 16h. (C-F) Control (Glut1fl/fl) and CD4CreGlut1fl/fl T cells were rested in IL-7 or CD3/CD28-stimulated for 16h. (C) Total lactate produced was measured. (D) Extracellular acidification rate (ECAR) was assessed after the addition of glucose (gluc), oligomycin (oligo), and 2-deoxyglucose (2-DG) at indicated times and (E) glycolytic capacity and (F) Oxygen Consumption Rate (OCR)/ECAR ratio determined. Mean ± SD (n=4) are shown from a minimum of 2 or more independent experiments.
Activated human T cells require metabolic reprogramming through Glut1
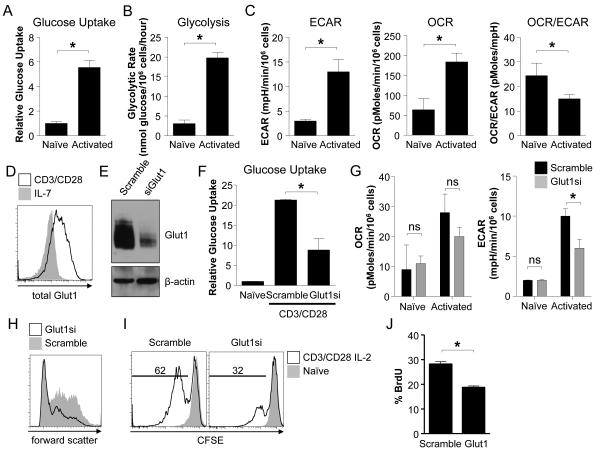
Despite evidence of murine T cell reliance on increased glucose uptake and glycolysis after stimulation (Frauwirth et al., 2002; Jacobs et al., 2008; Michalek et al., 2011a; Wang et al., 2011), metabolic transitions and dependencies in human T cells (hT cells) are poorly described. To test if a transition from an oxidative to glycolytic metabolism with increased Glut1 was also critical in human T cell activation, peripheral blood hT cells from healthy donors were stimulated with anti-CD3 and anti-CD28 for 48h. hT cell activation resulted in increased glucose uptake and glycolytic rate (Fig. 5A, B). ECAR and OCR also increased, although the ratio of OCR to ECAR decreased following CD3 and CD28 stimulation, indicating metabolic reprogramming towards aerobic glycolysis (Fig. 5C). Glut1 was also strongly upregulated (Fig. 5D).
Figure 5. Activation of human T cells triggers Glut1-dependent glycolytic reprogramming.
(A-D) Isolated human T cells were rested in IL-7 (naïve) or CD3/CD28-stimulated for 48 hours to measure (A) glucose uptake, (B) glycolytic flux, (C) ECAR and OCR, and (D) Glut1 expression by flow cytometry. (E-G) T cells transfected with scrambled or Glut1-targeted siRNA pools were cultured in IL-7 (naïve) or CD3/CD28-stimulated 48h to measure (E) Glut1 by immunoblot, (F) glucose uptake, (G) and OCR and ECAR. (H-I) T cells were transfected as above, CFSE-labeled and rested in IL-7 (naïve) or CD3/CD28-stimulated for 72h in the presence of IL-2. Flow cytometry measured (H) cell size by forward scatter and (I) proliferation by CFSE dilution. (J) T cells were transfected and stimulated and Bromodeoxyuridine (BrdU) was added for the final 8h of culture. BrdU incorporation was assessed by flow cytometry. Data show mean ± SD and are representative of (A-J) 3-5 or (J) two independent experiments.
To test the dependence of hT cells on Glut1 and glycolytic reprogramming, glucose metabolism was targeted pharmacologically and genetically. Despite the availability of alternate carbon sources, glucose-deprived or 2-deoxyglucose (2DG)-treated hT cells were unable to grow, upregulate the activation markers CD25, CD71, or proliferate (Figs. S4A, B). The role of Glut1 upregulation was then tested by transfection of Glut1 or scramble siRNA pools and knockdown was validated using semi-quantitative RT-PCR and immunoblot (Fig. 5E, S4C). Glut1 siRNA transfected hT cells had significantly lower ability to uptake glucose and had reduced ECAR after stimulation relative to control siRNA transfected cells (Fig. 5G). While Glut1 knockdown did not affect resting hT cell survival, Glut1-deficient hT cells had a modest yet significant reduction in viability when stimulated (Fig. S4D). Importantly, Glut1 siRNA transfected hT cells also had reduced ability to grow (Fig. 5H) and proliferate (Fig. 5I, J) upon stimulation. Thus, activated human T cells require Glut1 upregulation to support metabolic reprogramming for optimal survival, growth, and proliferation.
Glut1 is required for Teff, but not nTreg or iTreg, generation
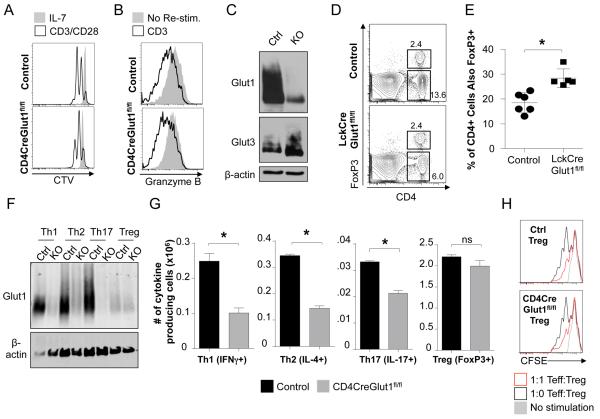
We and others have previously shown in vitro that murine Teff (Th1, Th2, and Th17) utilize a highly glycolytic metabolism while Treg are primarily oxidative and use lipids as a fuel (Michalek et al., 2011a; Shi et al., 2011). Likewise, effector CD8 T cells are glycolytic whereas memory CD8 T cells utilize lipid oxidation (Gubser et al., 2013; Pearce et al., 2009; van der Windt et al., 2012). We first tested the role of Glut1 in CD8 T cell activation and effector differentiation. While CD4CreGlut1fl/fl CD8 T cells had reduced initial proliferation (Fig. 6A), differentiation to effector function and the ability to express and release granzyme B upon stimulation or to express IL-2, TNFα and IFNγ were normal (Fig. 6B, S5A). Normal function of CD8 effectors was not due to failure of Glut1-deletion, as CD4CreGlut1fl/fl CD8 T cells have sharply reduced Glut1 protein (Fig. 6C). Rather, CD8 T cells may utilize alternate glucose transporters or metabolic programs, as CD4CreGlut1fl/fl CD8T cells had increased Glut3 and Glut6 expression (Fig. 6C, S5B).
Figure 6. Glut1 is required for Teff, but not Treg or CTL, generation and function.
(A) CD8 T cells from control (Glut1fl/+) and CD4CreGlut1fl/fl mice were CellTrace Violet (CTV) labeled and rested in IL-7 or CD3/CD28-stimulated + IL-2 for flow cytometric analysis after 48 hours. (B, C) Control (Glut1fl/+) and CD4CreGlut1fl/fl cytotoxic CD8 T lymphocytes (CTL) were analyzed (B) after 3h restimulation by flow cytometry and (C) immunoblot. (D, E) Flow cytometry of control (Glut1fl/fl) and LckCreGlut1fl/fl spleen for FoxP3+ CD4 T cells. (D) Representative plot and (E) cumulative data of fraction of CD4 T cells expressing FoxP3. (F-H) Th1, Th2, Th17, and Treg were induced using control (Glut1fl/+, Glut1fl/fl) and CD4CreGlut1fl/fl CD4 T cells and analyzed by (F) immunoblot, (G) flow cytometry to determine the number of live skewed cells expressing indicated subset markers. (H) Treg function was tested in an in vitro suppression assay. Data are representative or show mean cell count ± SD from (D, E) 5, (F-H) 3, or (A-C) 2 independent experiments.
Control and Glut1-deficient Teff and Treg were next examined to test the selective dependence of CD4 subsets on Glut1. Interestingly, while overall peripheral T cell numbers and frequency were lower in the spleen of LckCreGlut1fl/fl mice, the CD4+ FoxP3+ natural Treg (nTreg) population was not decreased and only FoxP3 negative cells were reduced (Fig. 6D). This selective loss of FoxP3- cells led to a progressive increase in representation of FoxP3+ nTreg in the peripheral CD4 T cell compartment (Fig. 6E). Naïve CD4 T cells can be induced to differentiate into Th1, Th2, Th17, or Treg subsets in appropriate cytokine conditions (Zhu et al., 2010). CD4 T cells from control and CD4CreGlut1fl/fl mice were therefore activated in vitro under polarizing conditions to generate Teff and Treg. The addition of cytokines reduced activation-induced cell death, allowing generation of each subset. The surviving cells in each case remained Glut1-deficient (Fig. 6F). Importantly, the number of cytokine producing cells in each Teff culture and the fraction of viable cytokine producing cells in Th1 and Th17 cultures were reduced by Glut1-deficiency (Fig. 6G, S5C, D). The total number of Teff in each condition was also reduced (Fig. S5D). Conversely, induced Treg cultures were unaffected by Glut1-deletion and maintained normal cell numbers, fraction positive for FoxP3, and ability to suppress Teff proliferation (Fig. 6G, H, S5C, D). The normal number and function of Glut1-deficient Treg did not appear due to compensation by alternate glucose transporters, as Gluts 3 and 8 were unchanged and expressed at low levels and Glut6 was only modestly increased by Glut1-deletion (Fig. S5E). Rather, these data suggest that Teff, but not Treg, are dependent on Glut1 for expansion and survival.
Glut1 is required in vivo for Teff but not Treg expansion in inflammatory disease
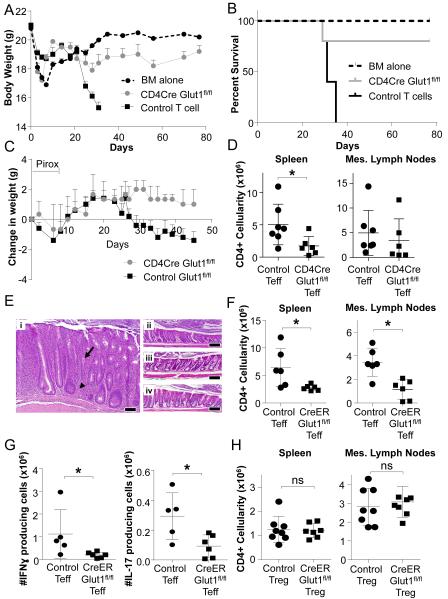
The Glut1-dependence of CD4 Teff may allow metabolic targeting in immunological disease and selective Teff dependence on Glut1 was tested in vivo. Acute GvHD is mediated through TCR recognition of allo-antigen and the role of glucose relative to lipid metabolism has been uncertain (Byersdorfer et al., 2013; Gatza et al., 2011; Saha et al., 2013). We first directly tested if T cells require Glut1 to induce GvHD by lethal irradiation followed by transplantation of T cell-depleted bone marrow alone or together with total control or CD4CreGlut1fl/fl T cells. Recipients were monitored over time for weight and survival. In support of a key role for T cell expression of Glut1 in this setting, allo-reactive Glut1-deficient T cells had dramatically decreased ability to induce lethal GvHD (Fig. 7A, B). Therefore, Glut1 is critical in T cell induction of GvHD.
Figure 7. Glut1 is selectively required for Teff, but not Treg, expansion and function in inflammatory disease.
(A, B) GvHD was induced with transplant of T cell-depleted bone marrow (BM) or BM + control (Glut1fl/fl or Glut1fl/+) or CD4CreGlut1fl/fl T cells into allogeneic hosts and (A) body weight and (B) survival were measured over time. (C-D) Naïve control (Glut1fl/fl) or CD4CreGlut1fl/fl T cells were transferred into Rag1−/− hosts and colitis was triggered 2 weeks later via piroxicam (Pirox; day 0 on start of Pirox) exposure and (C) animal weights measured over time, or (D) mice were sacrificed at day 30 and the number of CD4 T cells present in the spleen and mesenteric (mes) lymph nodes determined by flow cytometry. (E-H) Rag1−/− mice were injected with control (CreERGlut1+/+, Glut1fl/fl) or CreERGlut1fl/fl naïve T cells. Colitis was triggered by piroxicam exposure 2 weeks after T cell transfer. Animals were then treated with tamoxifen to activate Cre. (E) H&E histology of proximal colon from mice that received (i) naïve control T cells, (ii) naïve CreERGlut1fl/fl T cells, (iii) naïve control T cells plus control Treg, or (iv) naïve control T cells plus CreERGlut1fl/fl Treg. Bar indicates 100μm; arrow indicates cryptic abscess and arrowhead indicates a granuloma. (F) The number of CD4 or (G) IFNγ or IL-17 producing T cells in the mesenteric (mes) lymph nodes was determined after 4 weeks by flow cytometry. (H) Rag1−/− mice were co-injected with wild type naïve T cells and either control (CreERGlut1+/+, Glut1fl/fl) or CreERGlut1fl/fl nTreg. Mice were treated after 2 weeks with piroxicam and tamoxifen to trigger IBD and activate Cre and CD4 T cells were determined after 4 weeks. Data are representative of 2 (A-B) or 3 (C-H) independent experiments and show mean clinical score ± SEM (A, C) or mean ± SD (D, F-H).
Colitis is driven by Th1 and Th17 Teff and suppressed by Treg (Brand, 2009). The requirement of Teff for Glut1 to induce IBD was first tested using an adoptive transfer model (Mottet et al., 2003) in which sorted naïve control and CD4CreGlut1fl/fl T cells were transferred into immunodeficient Rag1−/− recipients. The NSAID piroxicam was given two weeks after T cell transfer to induce gut damage and trigger disease. Importantly, CD4CreGlut1fl/fl T cells were unable to effectively promote IBD as indicated by weight loss (Fig. 7C; day 0 indicates the start of piroxicam treatment), while control T cells induced significant weight loss over time. Importantly, both total (Fig. 7D, Fig. S6A) and cytokine producing (Fig. S6B, C) T cell numbers were decreased by Glut1-deficiency and the remaining T cells lacked Glut1 (Fig. S6D).
To test if acute deletion of Glut1 also affected IBD, sorted naïve T cells or nTreg from control or UbiCreERT2Glut1fl/fl mice were adoptively transferred individually or in combination into Rag1−/− hosts. After two weeks to allow homeostatic expansion, mice were treated with piroxicam to trigger IBD followed by tamoxifen to activate CreERT2 and delete Glut1 in Teff or Treg subsets in vivo. Although Glut1 deletion was incomplete (Fig. S7A), UbiCreERT2Glut1fl/fl T cells failed to induce inflammation, gut hyperplasia or granuloma, which were observed with control T cells (Figs. 7E panels i and ii). Broadly scoring colitis including architectural distortion, crypt abscesses, severity of inflammation, ulceration, and percent of bowel affected, suggested mice that received Glut1-deficient T cells were resistant to severe colitis (Fig. S7B). Importantly, total numbers of Glut1-deficient CD4 T cells in the spleen and mesenteric lymph nodes were significantly reduced relative to control T cells 4 weeks after tamoxifen treatment (Figs. 7F, S7C). Glut1-deletion also reduced cytokine production by CD4 Teff, as fewer IFNγ and IL-17-producing cells were present in mice receiving Glut1-deficient T cells (Fig. 7G, S7D, S7E).
Importantly, Glut1-deficient Treg appeared functional in vivo and capable of inhibiting Teff in IBD. Glut1-deficient nTreg suppressed Teff expansion similar to wild type nTreg, as total CD4+ cell numbers in the spleen and mesenteric lymph nodes and gut pathology were identical when control or Glut1-deficient nTreg were transferred together with control Teff cells (Fig. 7E panels iii and iv, 7H, S7F). Thus, while Teff populations require Glut1 expression to drive inflammatory colitis, in vitro and in vivo data support a model in which Treg appear capable to suppress Teff-mediated inflammation and expansion irrespective of Glut1 expression. Dependence of activated T cells and Teff on Glut1 may, therefore, allow selective targeting of T cell glucose metabolism to suppress inflammatory responses and promote tolerance and immune suppression.
DISCUSSION
The in vivo metabolic demands of T cell activation, proliferation, and differentiation and the programs that T cells initiate to support these needs may provide new targets to modulate the immune response (MacIver et al., 2013). In vivo, T cells require mitochondrial ROS (Sena et al., 2013), lipid synthesis (Kidani et al., 2013), and amino acid uptake (Sinclair et al., 2013). The mechanism and role of glucose uptake in T cell homeostasis, activation, and differentiation have not been directly tested in vivo. Here, our approach that directly targeted the first step of glucose metabolism by genetic deletion of Glut1 identified a selective reliance on this glucose transporter in T cell proliferation and CD4 Teff expansion to induce GvHD and colitis, while Treg appear largely Glut1-independent.
In addition to the cMyc (Wang et al., 2011) and Estrogen Related Receptor α (ERRα) (Michalek et al., 2011b) transcription factors that regulate T cell metabolism, mTOR and the PI3K-Akt-mTOR complex 1 (mTORC1) signaling pathway can promote Glut1 cell surface trafficking, glycolysis, and lipid synthesis (Duvel et al., 2010; Wieman et al., 2007) and Teff (Waickman and Powell, 2012). T cell-specific deletion of mTOR kinase to eliminate both mTORC1 and mTORC2 prevented generation of Teff, but allowed establishment of functional Treg (Delgoffe et al., 2009). Conversely, specific deletion of mTORC1 activation or components has been shown in distinct settings to not affect (Delgoffe et al., 2011) or to prevent (Zeng et al., 2013) Treg suppressive function. These data suggest that mTOR-driven glucose or lipid metabolism may be critical for Treg (Zeng et al., 2013). However, decreased mTORC1 signaling can lead to exacerbated mTORC2 activity (Zeng et al., 2013) that can also promote glycolytic metabolism (Gubser et al., 2013; Masui et al., 2013) and may thus suppress Treg activity. Indeed simultaneous deletion of essential mTORC1 and mTORC2 components or mTOR itself restored Treg function (Delgoffe et al., 2009; Zeng et al., 2013). This complex interplay between the multiple mTOR-induced signaling and metabolic events has hindered mechanistic interpretation of the specific role of metabolic regulation in T cell function and fate. Our studies directly test the role of Glut1 upregulation in T cell activation and subsets to show a selective Glut1-dependence of activated Teff and Glut1-independence of Treg.
Glucose uptake is mediated through the fourteen-member Glut transporter family, of which we show CD4 T cells express Gluts1, 3, 6, and 8. Each was regulated in T cell activation and differentiation, with highest expression of Glut1 and Glut3. Gluts 6 and 8 were expressed at relatively low levels and their specific roles are unclear at this time. The high Glut3 expression and Glut1-independence of resting T cells and CD8 Teff suggests a role for Glut3-mediated glucose uptake. While CD4 Teff cells were reliant on Glut1-directed metabolism, functional Treg could be generated from naïve T cells lacking Glut1, despite expressing only low levels of Glut3. These data are consistent with Treg use of mitochondrial oxidative pathways rather than glucose metabolism (Michalek et al., 2011a; Shi et al., 2011). In support of an alternate metabolic program, Treg can be generated in the absence of glucose (Michalek et al., 2011a) or in the presence of 2DG (Shi et al., 2011).
Despite expression of multiple glucose transporters, our data demonstrate a specific requirement for Glut1 in both activated mouse and human T cells in vitro and in vivo. The Glut1-dependent molecular switch to elevate glycolysis was critical for rapid human T cell growth and proliferation, as Glut1 knockdown suppressed glycolysis and slowed the transition of human T cells from quiescence to proliferation. Likewise, activation of murine cells led to a dependence on Glut1 to support cell growth, proliferation, and prevention of apoptosis. Thymocytes in the proliferative DN3-DN4 transition expressed Glut1 at a high level and were also sensitive to Glut1-deficiency. In the absence of Glut1, activated mature T cells failed to increase glucose uptake and glycolysis beyond resting levels, had selectively reduced growth and proliferation, and many cells underwent apoptosis. These broad inhibitory effects of Glut1-deficiency were potentially due to AMPK activation and suppression of mTORC1 in vitro and in vivo that resulted in fewer inflammatory cytokine-producing cells (Delgoffe et al., 2011; MacIver et al., 2011).
The differential requirements of specific T cell populations for Glut1 may reflect specific functional needs for a highly glycolytic metabolism. Aerobic glycolysis is closely linked with cell growth to generate increased mass for cell proliferation (Vander Heiden et al., 2009) and Glut1-deficient T cells failed to grow after stimulation. Proliferation was also suppressed and cell death increased, possibly as a consequence of inadequate nutrients to support biosynthesis and prevent AMPK suppression of mTORC1. In addition, Glut1-deficient CD4 Teff cells also had reduced production of IFNγ. Glucose-deprivation has been shown to lead to a specific reduction of IFNγ production in vitro (Cham and Gajewski, 2005; Jacobs et al., 2008) and glycolytic flux has been implicated in IFNγ translation (Chang et al., 2013). Indeed, we found both reduced cell numbers and decreased inflammatory cytokine production by Glut1-deficient Th1 and Th17. In contrast to Teff, our data show that Treg appear to utilize an alternate metabolic program. It remains to be determined, however, if Treg induce aerobic glycolysis using an alternate glucose transporter or if they proliferate using a distinct metabolic program. The metabolic requirements of different T cell activation states and subsets may also play a broad role in immune homeostasis or disease, supporting specific T cell populations in distinct tissues and immunologic settings. Together, our data demonstrate a primary role for Glut1 to support Teff expansion and survival.
Identifying biochemical requirements for T cell activation and the generation of effector and regulatory T cells has been a long sought goal in efforts to treat inflammatory diseases. Mechanisms that control cell metabolism to support the specific functional needs of these cells have been described only recently (MacIver et al., 2013), but have proven promising (Bian et al., 2009; Eleftheriadis et al., 2013; Ostroukhova et al., 2012; Shi et al., 2011). To date, pharmacologic approaches have provided limited mechanistic insight, and the role of glucose uptake has been uncertain. Data presented here demonstrate that despite expression of multiple Glut family transporters, Glut1 is specifically required for the cell-intrinsic metabolic program of activated T cells and CD4 Teff in vitro and in vivo to drive inflammation in both colitis and GvHD. These data show that despite a potential requirement for lipid oxidation (Byersdorfer et al., 2013; Gatza et al., 2011), Glut1 is central in the metabolism of Teff and in GvHD. Glut1, however, is not required in all settings, as resting T cells, CD8 Teff, and Treg were capable of Glut1-independent function. Collectively, these findings demonstrate that distinct T cell subsets utilize selective metabolic programs with differing dependence on Glut1. Understanding the roles and regulations of specific nutrient transporters in T cell activation and subsets may now provide new opportunities to exploit metabolic distinctions of cells in the immune system to control inflammatory diseases.
EXPERIMENTAL PROCEDURES
Human T cell Isolation, siRNA, Stimulation, and Culture
Human T cells were isolated by magnetic bead negative selection and cultured under standard conditions. Where indicated, cells were supplemented with 10mM 2-deoxyglucose (2DG) or cultured in glucose-free media, supplemented as indicated with D-glucose. Human siRNA pools were transiently transfected by nucleofection (Amaxa) and cells rested 4-6h before stimulation. Where indicated cells were activated on plates coated with anti-CD3 and anti-CD28, stimulated in the presence of 20ng/ml IL-2, or rested in 10ng/ml IL-7.
Mice
Mice were obtained from the Jackson laboratory or described previously (Young et al., 2011). All procedures were performed under Duke University Medical Center IACUC-approved protocols.
Murine T cell Isolation, Stimulation and Culture
Murine total, CD8 or CD4 T cells were isolated by magnetic bead negative selection and cultured under standard conditions. Where stated, cells were cultured in 10ng/ml IL-7 or activated by stimulation on plates coated with anti-CD3 and anti-CD28 or stimulated in the presence of 10μM LY294002, 20nM rapamycin, or 1μM PP242.
Metabolic Assays
Glycolysis and glucose uptake assays were normalized to cell number and have been described previously (Wieman et al., 2007). Oxygen consumption rate (OCR) and extracellular acidification rate (ECAR) were measured with an XF24 extracellular flux analyzer (Seahorse Bioscience) using manufacturer recommended protocols. For certain experiments, ECAR was measured over time following injection of 10mM D-glucose, 1μM oligomycin and 20mM 2DG. Lactate production was measured by colorimetric assay (Abcam).
Flow Cytometry, Proliferation and Viability Measurements
Intracellular cytokines were measured as described (Michalek et al., 2011a). To measure transcription factors, granzyme B, and phospho-S6, cells were fixed in paraformaldehyde, methanol permeabilized and then labeled with fluorophore-conjugated antibodies. Cell proliferation was assayed by flow cytometry of carboxyfluorescein succinimidyl ester (CFSE) or CellTrace Violet (CTV) labeled cells and viability was determined by propidium iodide exclusion (PI; Invitrogen). To assay bromodeoxyuridine (BrdU) incorporation cells were cultured with BrdU for 8h, ethanol fixed then stained with Alexa-Fluor 647 anti-BrdU (Invitrogen). Cells were labeled with PI for cell cycle analysis and analyzed as described (Darzynkiewicz and Juan, 2001).
T cell differentiation and Treg Suppressor Assay
Naïve CD4+CD25-T cells were used to generate subsets as described (Michalek et al., 2011a). To generate CD8+ cytotoxic T lymphocytes (CTL), CD8+ T cells were stimulated for 48h with anti-CD3 and anti-CD28 in the presence of 20ng/ml IL-2. Cells were then cultured with 20ng/ml IL-2 alone for a further 72h. Treg suppression was measured as described (Michalek et al., 2011a).
Glucose transporter family expression
RNA was isolated using a RNAeasy Plus Mini kit (Qiagen). Glut family absolute copy number was determined as described (Rudolph et al., 2011). Ct values were fitted to regression curves to quantify transcript copy number. RNA was reverse transcribed using a commercially available kit for semi-quantitative real time PCR performed using a fluorescent dsDNA dye and mRNA levels normalized to 18S RNA or β2 microglobin mRNA using the ΔΔCt method.
Immunoblotting
Immunoblotting was performed using standard techniques as described previously (Jacobs et al., 2008).
Homeostatic Proliferation
Total T cells were isolated from control and CD4CreGlut1fl/fl mice, labeled with CTV and adoptively transferred into irradiated Thy1.1 hosts (600cGy). Six days after transfer, lymphocytes were isolated and proliferation was assessed by CTV dilution of Thy1.2 labeled cells.
Ova Immunization
Ovalbumin (Ova)-specific OT-II T cell receptor (TCR) Thy1.2 transgenic control (CD4CreGlut+/+) or knockout (CD4CreGlut1fl/fl) CD4 T cells were labeled with CTV and adoptively transferred into wild type Thy1.1 hosts. One day later, mice were immunized with i.p. Ova in Complete Adjuvant (Sigma). After three days, lymphocyte proliferation was assessed by CTV dilution of Thy1.2 labeled cells.
Graft Versus Host Disease
Lethally irradiated (8.5 Gy) BALB/c mice were injected intravenously with 1×107 T cell depleted bone marrow (TCD BM) cells along with 1×106 purified T cells from allogeneic donors. Body weight, survival, and clinical evidence of GvHD such as skin changes, diarrhea, hunched posture, and activity were closely monitored after transplantation as described (Chen et al., 2003).
Colitis
Sorted naïve effector T cells (CD4+CD25-CD45RBhi) were injected i.p. into C57BL/6 RAG1−/− recipients. Sorted Treg (CD4+CD25+CD45RBlo) cells were co-injected as indicated. Because the mice were free of enteric pathogens including helicobacter species and colitis may not occur spontaneously in this setting, mice were fed 200ppm piroxicam in powdered rodent chow for 5 days two weeks after T cell injection to increase gut permeability and trigger colitis (Hale et al., 2005). Mice were then injected i.p with 4mg/kg/day of tamoxifen for 4 days to induce Glut1fl deletion and monitored three times weekly. Colon tissue was isolated for pathology analysis and disease scoring as described (Hale et al., 2005 Hale et al., 2005).
Statistical Analysis
Statistical analyses were performed with Prism software (GraphPad) using the nonparametric Mann Whitney test. The Wilcoxon signed-rank test was used for paired samples. Longitudinal data was analyzed by two-way ANOVA followed by Tukey’s test. Statistically significant results are indicated (* p < 0.05) and n.s. indicates select non-significant data. Error bars show mean ± SD unless otherwise indicated to show mean ± SEM.
Supplementary Material
HIGHLIGHTS.
CD4 T cells express multiple glucose transporters, including Gluts 1, 3, 6, and 8
Glut1 has non-redundant function in activated, but not resting CD4 T cells
CD4 Th1 and Th17 selectively require Glut1 in vivo to regulate immunologic diseases
Targeting T cell glucose metabolism in vivo can selectively impact effector cells
ACKNOWLEDGMENTS
We thank the members of the Rathmell laboratory for helpful discussions. This work was supported by the American Asthma Foundation (J.C.R.), the Leukemia Lymphoma Society (J.C.R.), the Crohn’s and Colitis Foundation of America (284879, A.N.M.), the Kenneth Rainin Foundation (J.C.R.), and R01AI063345 (J.C.R.), R01HL108006 (J.C.R.), R56AI102074 (J.C.R.), RO1CA138482 (S.M.A.), and UO1HL087947 (E.D.A.), and P01CA047741 (B.J.C.) from the NIH.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Supplemental information contains seven figures, legends, and detailed experimental methods.
AUTHOR CONTRIBUTIONS
J.C.R., A.N.M and V.A.G designed the study and wrote the manuscript; A.N.M., V.A.G, A.G.N, R.D.M, M.C.R and D.D. performed the experiments; L.P.H and B.J.C analyzed data; E.D.A provided mice and reviewed the manuscript; S.M.A, B.J.C and L.P.H gave technical support and conceptual advice.
REFERENCES
- Bian L, Josefsson E, Jonsson IM, Verdrengh M, Ohlsson C, Bokarewa M, Tarkowski A, Magnusson M. Dichloroacetate alleviates development of collagen II-induced arthritis in female DBA/1 mice. Arthritis research & therapy. 2009;11:R132. doi: 10.1186/ar2799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brand S. Crohn’s disease: Th1, Th17 or both? The change of a paradigm: new immunological and genetic insights implicate Th17 cells in the pathogenesis of Crohn’s disease. Gut. 2009;58:1152–1167. doi: 10.1136/gut.2008.163667. [DOI] [PubMed] [Google Scholar]
- Byersdorfer CA, Tkachev V, Opipari AW, Goodell S, Swanson J, Sandquist S, Glick GD, Ferrara JL. Effector T cells require fatty acid metabolism during murine graft-versus-host disease. Blood. 2013;122:3230–3237. doi: 10.1182/blood-2013-04-495515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carrette F, Surh CD. IL-7 signaling and CD127 receptor regulation in the control of T cell homeostasis. Semin Immunol. 2012;24:209–217. doi: 10.1016/j.smim.2012.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cham CM, Gajewski TF. Glucose availability regulates IFN-gamma production and p70S6 kinase activation in CD8+ effector T cells. J Immunol. 2005;174:4670–4677. doi: 10.4049/jimmunol.174.8.4670. [DOI] [PubMed] [Google Scholar]
- Chang CH, Curtis JD, Maggi LB, Jr., Faubert B, Villarino AV, O’Sullivan D, Huang SC, van der Windt GJ, Blagih J, Qiu J, et al. Posttranscriptional control of T cell effector function by aerobic glycolysis. Cell. 2013;153:1239–1251. doi: 10.1016/j.cell.2013.05.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen BJ, Cui X, Sempowski GD, Chao NJ. Growth hormone accelerates immune recovery following allogeneic T-cell-depleted bone marrow transplantation in mice. Exp Hematol. 2003;31:953–958. doi: 10.1016/s0301-472x(03)00196-6. [DOI] [PubMed] [Google Scholar]
- Darzynkiewicz Z, Juan G. Robinson J. Paul, et al., editors. Analysis of DNA content and BrdU incorporation. Current protocols in cytometry/editorial board. 2001 doi: 10.1002/0471142956.cy0707s02. Chapter 7, Unit 7 7. [DOI] [PubMed] [Google Scholar]
- Delgoffe GM, Kole TP, Zheng Y, Zarek PE, Matthews KL, Xiao B, Worley PF, Kozma SC, Powell JD. The mTOR kinase differentially regulates effector and regulatory T cell lineage commitment. Immunity. 2009;30:832–844. doi: 10.1016/j.immuni.2009.04.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delgoffe GM, Pollizzi KN, Waickman AT, Heikamp E, Meyers DJ, Horton MR, Xiao B, Worley PF, Powell JD. The kinase mTOR regulates the differentiation of helper T cells through the selective activation of signaling by mTORC1 and mTORC2. Nat Immunol. 2011;12:295–303. doi: 10.1038/ni.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duvel K, Yecies JL, Menon S, Raman P, Lipovsky AI, Souza AL, Triantafellow E, Ma Q, Gorski R, Cleaver S, et al. Activation of a metabolic gene regulatory network downstream of mTOR complex 1. Mol Cell. 2010;39:171–183. doi: 10.1016/j.molcel.2010.06.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eleftheriadis T, Pissas G, Karioti A, Antoniadi G, Antoniadis N, Liakopoulos V, Stefanidis I. Dichloroacetate at therapeutic concentration alters glucose metabolism and induces regulatory T-cell differentiation in alloreactive human lymphocytes. Journal of basic and clinical physiology and pharmacology. 2013;24:271–276. doi: 10.1515/jbcpp-2013-0001. [DOI] [PubMed] [Google Scholar]
- Frauwirth KA, Riley JL, Harris MH, Parry RV, Rathmell JC, Plas DR, Elstrom RL, June CH, Thompson CB. The CD28 signaling pathway regulates glucose metabolism. Immunity. 2002;16:769–777. doi: 10.1016/s1074-7613(02)00323-0. [DOI] [PubMed] [Google Scholar]
- Gatza E, Wahl DR, Opipari AW, Sundberg TB, Reddy P, Liu C, Glick GD, Ferrara JL. Manipulating the bioenergetics of alloreactive T cells causes their selective apoptosis and arrests graft-versus-host disease. Science translational medicine. 2011;3:67ra68. doi: 10.1126/scitranslmed.3001975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gubser PM, Bantug GR, Razik L, Fischer M, Dimeloe S, Hoenger G, Durovic B, Jauch A, Hess C. Rapid effector function of memory CD8+ T cells requires an immediate-early glycolytic switch. Nat Immunol. 2013;14:1064–1072. doi: 10.1038/ni.2687. [DOI] [PubMed] [Google Scholar]
- Hale LP, Gottfried MR, Swidsinski A. Piroxicam treatment of IL-10-deficient mice enhances colonic epithelial apoptosis and mucosal exposure to intestinal bacteria. Inflammatory bowel diseases. 2005;11:1060–1069. doi: 10.1097/01.mib.0000187582.90423.bc. [DOI] [PubMed] [Google Scholar]
- Jacobs SR, Herman CE, Maciver NJ, Wofford JA, Wieman HL, Hammen JJ, Rathmell JC. Glucose Uptake Is Limiting in T Cell Activation and Requires CD28-Mediated Akt-Dependent and Independent Pathways. J Immunol. 2008;180:4476–4486. doi: 10.4049/jimmunol.180.7.4476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobs SR, Michalek RD, Rathmell JC. IL-7 is essential for homeostatic control of T cell metabolism in vivo. J Immunol. 2010;184:3461–3469. doi: 10.4049/jimmunol.0902593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kidani Y, Elsaesser H, Hock MB, Vergnes L, Williams KJ, Argus JP, Marbois BN, Komisopoulou E, Wilson EB, Osborne TF, et al. Sterol regulatory element-binding proteins are essential for the metabolic programming of effector T cells and adaptive immunity. Nat Immunol. 2013;14:489–499. doi: 10.1038/ni.2570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacIver NJ, Blagih J, Saucillo DC, Tonelli L, Griss T, Rathmell JC, Jones RG. The liver kinase B1 is a central regulator of T cell development, activation, and metabolism. J Immunol. 2011;187:4187–4198. doi: 10.4049/jimmunol.1100367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacIver NJ, Michalek RD, Rathmell JC. Metabolic regulation of T lymphocytes. Annu Rev Immunol. 2013;31:259–283. doi: 10.1146/annurev-immunol-032712-095956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masui K, Tanaka K, Akhavan D, Babic I, Gini B, Matsutani T, Iwanami A, Liu F, Villa GR, Gu Y, et al. mTOR Complex 2 Controls Glycolytic Metabolism in Glioblastoma through FoxO Acetylation and Upregulation of c-Myc. Cell Metab. 2013;18:726–739. doi: 10.1016/j.cmet.2013.09.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCracken AN, Edinger AL. Nutrient transporters: the Achilles’ heel of anabolism. Trends Endocrinol Metab. 2013;24:200–208. doi: 10.1016/j.tem.2013.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michalek RD, Gerriets VA, Jacobs SR, Macintyre AN, Maciver NJ, Mason EF, Sullivan SA, Nichols AG, Rathmell JC. Cutting Edge: Distinct Glycolytic and Lipid Oxidative Metabolic Programs Are Essential for Effector and Regulatory CD4+ T Cell Subsets. J Immunol. 2011a;186:3299–3303. doi: 10.4049/jimmunol.1003613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michalek RD, Gerriets VA, Nichols AG, Inoue M, Kazmin D, Chang CY, Dwyer MA, Nelson ER, Pollizzi KN, Ilkayeva O, et al. Estrogen-related receptor-alpha is a metabolic regulator of effector T-cell activation and differentiation. Proc Natl Acad Sci U S A. 2011b;108:18348–18353. doi: 10.1073/pnas.1108856108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mottet C, Uhlig HH, Powrie F. Cutting edge: cure of colitis by CD4+CD25+ regulatory T cells. J Immunol. 2003;170:3939–3943. doi: 10.4049/jimmunol.170.8.3939. [DOI] [PubMed] [Google Scholar]
- Ostroukhova M, Goplen N, Karim MZ, Michalec L, Guo L, Liang Q, Alam R. The role of low-level lactate production in airway inflammation in asthma. American journal of physiology. Lung cellular and molecular physiology. 2012;302:L300–307. doi: 10.1152/ajplung.00221.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pearce EL, Walsh MC, Cejas PJ, Harms GM, Shen H, Wang LS, Jones RG, Choi Y. Enhancing CD8 T-cell memory by modulating fatty acid metabolism. Nature. 2009;460:103–107. doi: 10.1038/nature08097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rathmell JC, Farkash EA, Gao W, Thompson CB. IL-7 enhances the survival and maintains the size of naive T cells. J Immunol. 2001;167:6869–6876. doi: 10.4049/jimmunol.167.12.6869. [DOI] [PubMed] [Google Scholar]
- Rudolph MC, Russell TD, Webb P, Neville MC, Anderson SM. Prolactin-mediated regulation of lipid biosynthesis genes in vivo in the lactating mammary epithelial cell. Am J Physiol Endocrinol Metab. 2011;300:E1059–1068. doi: 10.1152/ajpendo.00083.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saha A, Aoyama K, Taylor PA, Koehn BH, Veenstra RG, Panoskaltsis-Mortari A, Munn DH, Murphy WJ, Azuma M, Yagita H, et al. Host programmed death ligand 1 is dominant over programmed death ligand 2 expression in regulating graft-versus-host disease lethality. Blood. 2013;122:3062–3073. doi: 10.1182/blood-2013-05-500801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sena LA, Li S, Jairaman A, Prakriya M, Ezponda T, Hildeman DA, Wang CR, Schumacker PT, Licht JD, Perlman H, et al. Mitochondria are required for antigen-specific T cell activation through reactive oxygen species signaling. Immunity. 2013;38:225–236. doi: 10.1016/j.immuni.2012.10.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi LZ, Wang R, Huang G, Vogel P, Neale G, Green DR, Chi H. HIF1alpha-dependent glycolytic pathway orchestrates a metabolic checkpoint for the differentiation of TH17 and Treg cells. J Exp Med. 2011;208:1367–1376. doi: 10.1084/jem.20110278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shortman K, Egerton M, Spangrude GJ, Scollay R. The generation and fate of thymocytes. Semin Immunol. 1990;2:3–12. [PubMed] [Google Scholar]
- Sinclair LV, Rolf J, Emslie E, Shi YB, Taylor PM, Cantrell DA. Control of amino-acid transport by antigen receptors coordinates the metabolic reprogramming essential for T cell differentiation. Nat Immunol. 2013;14:500–508. doi: 10.1038/ni.2556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thorens B, Mueckler M. Glucose transporters in the 21st Century. Am J Physiol Endocrinol Metab. 2010;298:E141–145. doi: 10.1152/ajpendo.00712.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Windt GJ, Everts B, Chang CH, Curtis JD, Freitas TC, Amiel E, Pearce EJ, Pearce EL. Mitochondrial respiratory capacity is a critical regulator of CD8+ T cell memory development. Immunity. 2012;36:68–78. doi: 10.1016/j.immuni.2011.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vander Heiden MG, Cantley LC, Thompson CB. Understanding the Warburg effect: the metabolic requirements of cell proliferation. Science. 2009;324:1029–1033. doi: 10.1126/science.1160809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waickman AT, Powell JD. mTOR, metabolism, and the regulation of T-cell differentiation and function. Immunol Rev. 2012;249:43–58. doi: 10.1111/j.1600-065X.2012.01152.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang RN, Dillon CP, Shi LZ, Milasta S, Carter R, Finkelstein D, McCormick LL, Fitzgerald P, Chi HB, Munger J, Green DR. The Transcription Factor Myc Controls Metabolic Reprogramming upon T Lymphocyte Activation. Immunity. 2011;35:871–882. doi: 10.1016/j.immuni.2011.09.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wieman HL, Wofford JA, Rathmell JC. Cytokine stimulation promotes glucose uptake via phosphatidylinositol-3 kinase/Akt regulation of Glut1 activity and trafficking. Mol Biol Cell. 2007;18:1437–1446. doi: 10.1091/mbc.E06-07-0593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young CD, Lewis AS, Rudolph MC, Ruehle MD, Jackman MR, Yun UJ, Ilkun O, Pereira R, Abel ED, Anderson SM. Modulation of glucose transporter 1 (GLUT1) expression levels alters mouse mammary tumor cell growth in vitro and in vivo. PLoS One. 2011;6:e23205. doi: 10.1371/journal.pone.0023205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeng H, Yang K, Cloer C, Neale G, Vogel P, Chi H. mTORC1 couples immune signals and metabolic programming to establish T(reg)-cell function. Nature. 2013;499:485–490. doi: 10.1038/nature12297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu J, Yamane H, Paul WE. Differentiation of effector CD4 T cell populations. Annu Rev Immunol. 2010;28:445–489. doi: 10.1146/annurev-immunol-030409-101212. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.