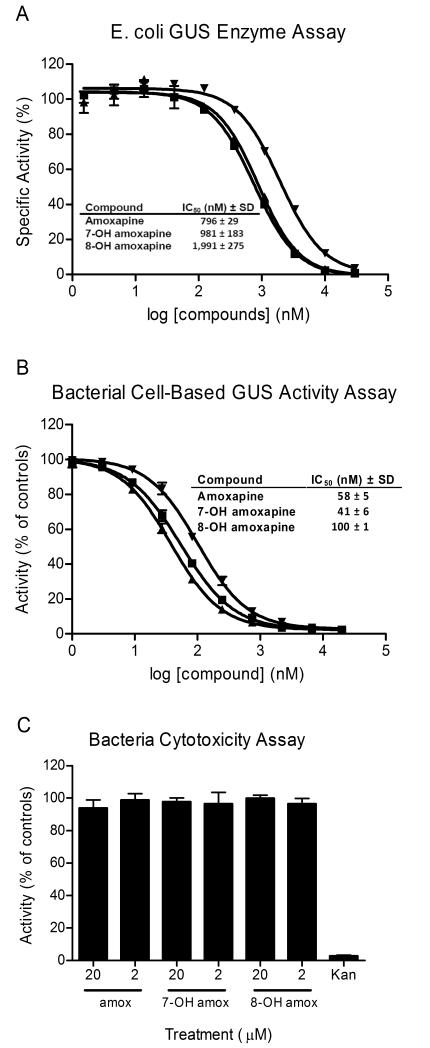
Fig. 3. In vitro potency determinations for amoxapine and its metabolites.
IC50 values provided in the tables are average values based on three independent determinations along with standard deviations (SD). (A) IC50 value determinations were performed for amoxapine (■), 7-OH amoxapine (▲) and 8-OH amoxapine (▼) using the GUS enzyme assay. The indicated concentrations were tested in duplicate and activity was normalized to control wells (± enzyme). (B) IC50 value determinations were performed for amoxapine (■), 7-OH amoxapine (▲) and 8-OH amoxapine (▼) using the bacterial cell-based GUS activity assay. The indicated concentrations were tested in duplicate and activity was normalized to control wells (± bacteria). (C) The indicated compounds were tested in the bacterial cytotoxicity assay at 20 and 2 μM, as indicated. The compounds were tested in triplicate at these concentrations and activity was normalized to control wells (± bacteria). The antibiotic kanamycin (Kan) was included as a positive control. Data points represent the average of two or three determinations per variable and error bars represent SD. Data shown are representative of three independent experiments.