Abstract
Motor learning and functional recovery from brain damage involve changes in the strength of synaptic connections between neurons. Relevant in vivo evidence on the underlying cellular mechanisms remains limited and indirect. We found that the strength of neural connections between motor cortex and spinal cord in monkeys can be modified with an autonomous recurrent neural interface that delivers electrical stimuli in the spinal cord triggered by action potentials of corticospinal cells during free behavior. The activity-dependent stimulation modified the strength of the terminal connections of single corticomotoneuronal cells, consistent with a bidirectional spike-timing dependent plasticity rule previously derived from in vitro experiments. For some cells the changes lasted for days after the end of conditioning, but most effects eventually reverted to preconditioning levels. These results provide the first direct evidence of corticospinal synaptic plasticity in vivo at the level of single neurons induced by normal firing patterns during free behavior.
Introduction
Synaptic plasticity is a key mechanism mediating reorganization of neural circuits during learning and recovery from injury (Cramer et al., 2011; Kleim, 2008; Wolpaw and Tennissen, 2001). In vitro studies have shown that the synaptic connection between two neurons can be strengthened or weakened when the presynaptic neuron fires before or after the postsynaptic neuron, as described by spike-timing dependent plasticity (STDP) rules (Bi and Poo, 2001; Caporale and Dan, 2008; Markram et al., 2011). Most of these studies have involved slice preparations of cerebral or hippocampal cortex, and elicited STDP using controlled stimulation patterns that differ from those typically occurring during normal behavior. Such studies have revealed that STDP rules for different synapses can differ with regard to polarity of the synaptic changes and width of the effective windows (Caporale and Dan, 2008). Moreover, the STDP rule for a given synapse can be significantly altered by the presence and concentration of neuromodulators (Salgado et al.; Seol et al., 2007). STDP is typically documented with repetitive pairing of pre- and post-synaptic spikes at regular intervals, but when tested with more complex spike patterns, STDP is a nonlinear and variable function of the pattern of pre- and postsynaptic activity (Froemke et al., 2010). In vitro experiments have confirmed that the conditioned effects can last for up to several hours in the absence of additional activity. These observations raise several questions addressed in this study: Can plasticity of primate corticospinal cell terminals be induced during free behavior by using the cell’s normal firing patterns to trigger stimulation of target motoneurons? What are the STDP rules pertaining to monosynaptic and disynaptic connections of primate corticomotoneuronal cells? How long do the conditioned effects last after the end of conditioning and the resumption of normal neuronal activity?
These questions were addressed by establishing an artificial connection between cortical neurons and spinal cord with a recurrent brain-computer interface. Experiments using a similar paradigm to deliver spike-triggered stimulation within motor cortex produced synaptic plasticity for spike-stimulus delays less than about 50 ms (Jackson et al., 2006a; Rebesco et al., 2010). In these studies the conditioning effects were documented indirectly, through changed outputs evoked by intracortical microstimulation (Jackson et al., 2006a) or through inferred functional interactions between the recorded populations (Rebesco et al., 2010). Since stimuli could only be delivered after the triggering spikes, only the facilitation phase of the STDP function was explored. Here we report the first direct evidence of changes in the synaptic strength of single corticospinal cells in monkeys induced by spike-triggered spinal simulation through an autonomous recurrent neural interface operating during free behavior. The finite conduction time from cortex to spinal cord allowed stimuli to be delivered before the arrival of the corticospinal impulses, which produced decreases in synaptic strength, consistent with a bidirectional STDP rule.
Results
Corticomotoneuronal (CM) cells, which connect cerebral cortex to spinal motoneurons, are an important subset of primate motor cortical neurons, playing a crucial role in fine control of digits (Porter and Lemon, 1993). Their output effects on target motoneurons can be documented in spike-triggered averages (SpTA) of rectified electromyographic (EMG) activity and their response properties documented during performance of motor tasks (Fetz and Cheney, 1980; Jackson et al., 2003; Schieber and Rivlis, 2005). For the first time we have recorded the activity of the same CM cells over successive days of free behavior, using an implanted electrode array (Jackson and Fetz, 2007) positioned to record CM cells located along the bank of the precentral gyrus (Rathelot and Strick, 2006; Smith and Fetz, 2009). The spinal cord was stimulated with a second set of microwires implanted at fixed depths in the cervical enlargement (Mushahwar and Horch, 1998). An artificial corticospinal connection between a CM cell and its target spinal site was produced with an autonomous head-fixed neural interface that operated continuously during free behavior in the monkeys’ home cage (Jackson et al., 2007; Jackson et al., 2006b). The so-called “Neurochip” was programmed to detect action potentials of a single CM cell and deliver an intraspinal stimulus after a fixed delay. We chose a spinal site where trains of stimuli evoked responses in one or more of the CM cell’s target muscles (Fig. 1A and B), thus confirming the activation of the cell’s postsynaptic motoneurons. During conditioning the spike-triggered spinal stimuli were above threshold for activating motoneurons, but too weak to disrupt normal movements or to activate the recorded CM cell, either antidromically or orthodromically (Fig. 1B).
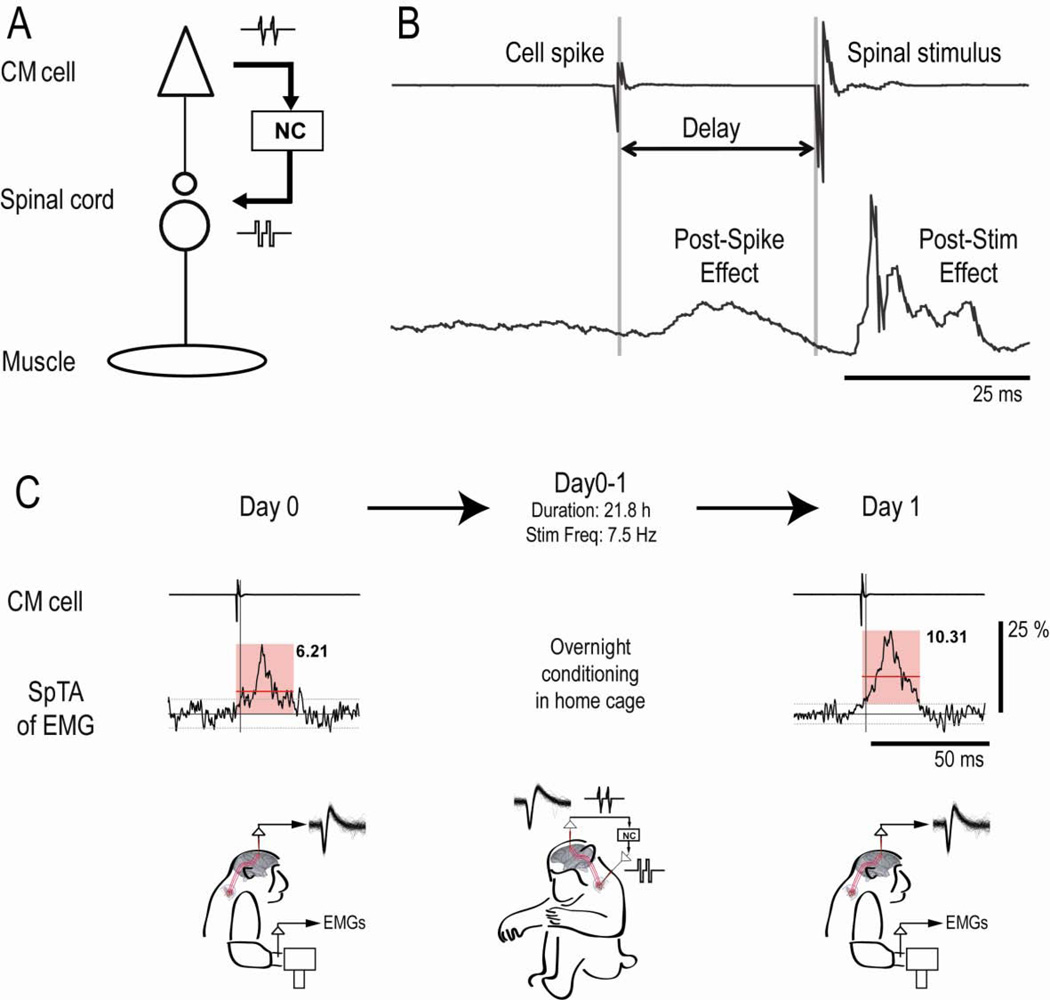
Figure 1. Experimental design and protocol.
A: Schematic showing action potentials of CM cell triggering intraspinal stimuli via neurochip (NC). B: Cortical recording (top) and SpTA of EMG (bottom) for CM spikes followed after delay of 25 ms by spinal stimulus. SpTA shows post-spike facilitation and post-stimulus response in same target muscle. C: SpTAs of EMG acquired before (Day 0) and after (Day 1) a 22h period of conditioning, showing analysis interval (pink square), baseline ± 2 SD of SpTA (horizontal gray lines), and MPI above baseline of feature (horizontal red lines and black numbers). Conditioning increased MPI by 66% (p=0.0003). Vertical bar calibrates 25% of baseline. Drawings represent monkey performing task on Days 0 and 1, and behaving freely during 21.8 h of conditioning, with mean spike frequency of 7.5 Hz.
The synaptic efficacy of the CM cells’ corticospinal terminals was assessed by the magnitude of spike-related effects (SpREs) in SpTAs, compiled while the monkey performed a standard isometric wrist target-tracking task. The magnitudes of the SpREs can be a function of behavior and the amount of EMG activity (Bennett and Lemon, 1994; Davidson et al, 2007), so we compiled the SpTAs using spikes occurring during comparable levels of EMG activity. As discussed in the supplementary information, under these conditions the main determinant of changes in magnitude of PSpE is the strength of the mediating postsynaptic potentials. Excitatory SpREs consist predominantly of post-spike facilitation (PSpF), mediated by sufficiently strong synaptic connections of the triggering CM cell to spinal motoneurons and interneurons, and beginning at an appropriate latency after the spike (Fig. 1B and 2C) (Fetz and Cheney, 1980; Schieber and Rivlis, 2005). Some SpREs can also include an earlier component due to synchrony with other CM cells, as discussed below. The magnitude of the SpRE was quantified by the mean percent increase (MPI) of the feature above baseline (Fetz and Cheney, 1980), and was used to document the change in efficacy of corticospinal terminals after 3.5 – 62 hours of conditioning with spike-triggered stimulation (Fig. 1C). Post-spike suppression effects, as seen in ECR of Fig. 2A, are probably mediated by a disynaptic link via inhibitory interneurons, and their MPI is a negative number.
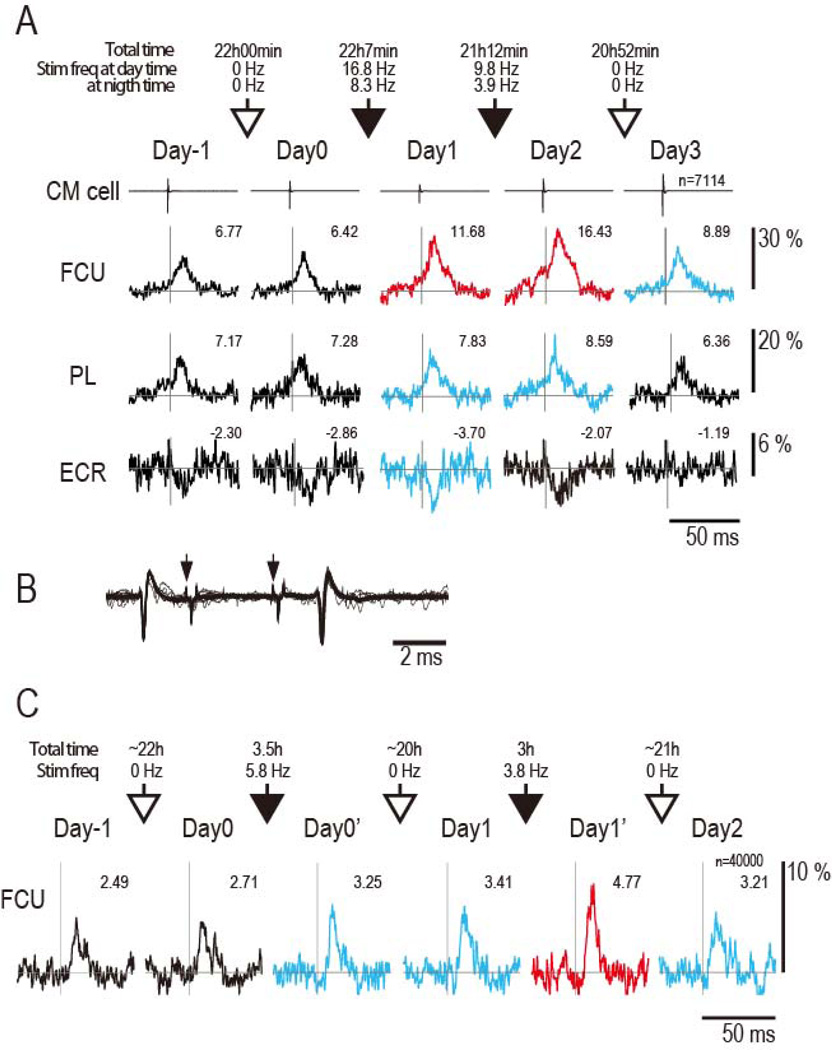
Figure 2. Strengthening of corticospinal connections.
A: SpTAs show PSpF in two flexor muscles and PSpS in an extensor. The PSpFs are superimposed on facilitatory synchrony effects in both flexor muscles. Numbers give MPI of the SpREs; vertical line corresponds to trigger event. First two columns show repeatability of SpREs for two successive days without intervening conditioning (Day -1 and Day 0). Open arrows designate periods without conditioning. Solid black arrows designate conditioning periods (50µA stimuli and 12 ms delay); duration and average stimulus frequencies given above arrows. Colors of SpTAs for subsequent days indicate significance of changes relative to Day 0: red: p< 0.01, blue p< 0.05, and black: no significant difference. Scales at right calibrate % of baseline. Numbers give MPI. FCU, flexor carpi ulnaris; PL, palmaris longus; ECR, extensor carpi radialis; n, number of triggering spikes for all SpTAs. B: Collision test for CM cell in A. Spontaneous spikes in cell triggered 2 spinal stimuli (arrows) with intensity sufficient to trigger antidromic spikes (seen after 2nd stimulus); antidromic response for 1st stimulus is absent due to collision. The current intensity (360µA) was higher than that used during conditioning. C: PSpEs for another CM cell, showing pure PSpF without synchrony. Conditioning times are 3.5 h on Day 0 and 3 h on day 1.
Strengthening of corticospinal connections through spike-triggered stimulation
Figure 2A illustrates the output effect in SpTAs of three muscles from a representative CM cell that had an excitatory effect on two flexor muscles and an inhibitory linkage to an extensor muscle. The magnitude and shape of SpREs from this CM cell were stable for two days prior to conditioning (cf. Day-1 and Day0). After the first 22 hours of spike-triggered stimulation, there was a statistically significant increase in all the SpREs, both facilitation and suppression (cf. Day1 vs Day0). The facilitation increased further after a second day of conditioning (Day2). The day after the end of conditioning (Day3) the SpREs in one muscle (FCU) remained above preconditioning levels.
The SpREs of CM cells could include “synchrony effects”, whose onsets were too early relative to the triggering spike to be mediated by the triggering cell, as seen in FCU and PL muscles of Fig. 2A. These early effects are mediated by other CM cells that are synchronized with the trigger cell. Cross-correlograms of synchronized CM cells typically show central peaks straddling the reference cell (Jackson et al., 2003; Smith and Fetz, 2009 and Supplementary Fig. S1B), so the average time of the synchronized spikes would be the same as those of the recorded CM cell. Thus, the conditioning paradigm would also strengthen terminals of the synchronized cells and lead to increases in the synchrony effects (see supplementary material for further discussion). Additional examples of increases in both PSpF and synchrony effects are shown in Supplementary Fig. S2.
To confirm that the terminals of the triggering CM cell alone could be strengthened, we also measured the effects of conditioning on a pure serial PSpF, shown in Fig. 2C. In this case the PSpF was re-measured after a relatively brief conditioning period of 3.5 hours, which proved sufficient to enhance the PSpF (cf. Day0’ with Day0). Moreover, in this case the increase in PSpF lasted over 24 hours following conditioning (cf. Day1 with Day0). These changes were confirmed a second time on the subsequent days after further conditioning (Day1 and Day2).
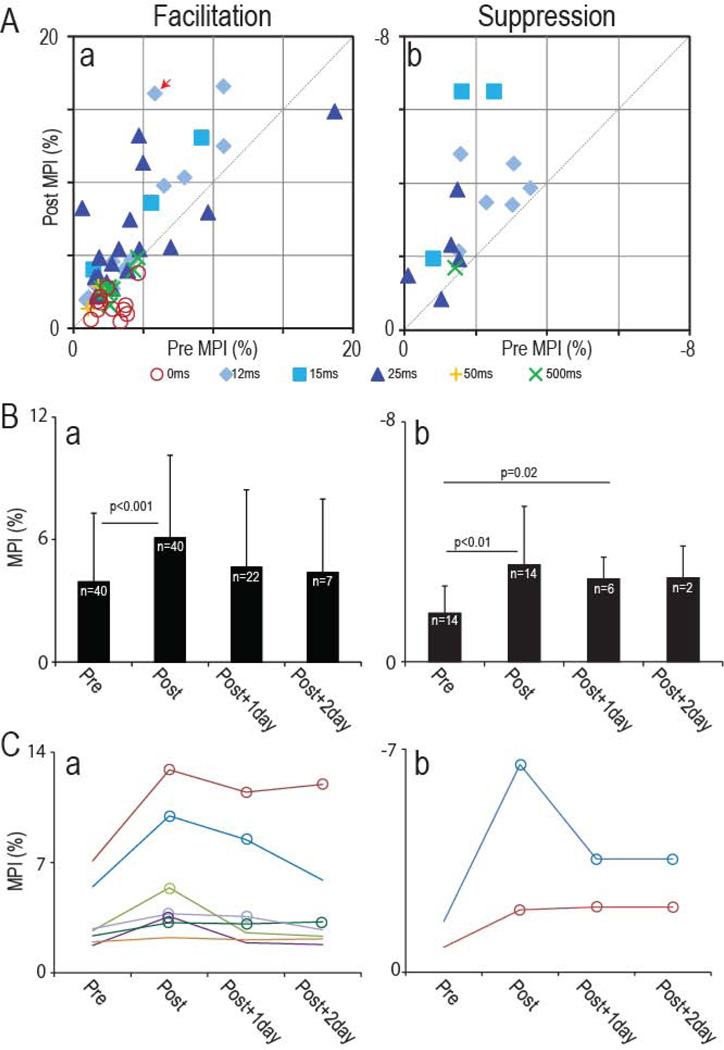
We investigated the effects of this conditioning paradigm in 19 different sessions, using 15 different pairs of CM cells and spinal sites in two monkeys. Conditioning produced significant changes in the 16 sessions that had appropriate spike-stimulus delays (for details of all sessions see Supplementary Table S1). The amplitudes of spike-related facilitation and suppression were both found to increase after overnight conditioning with spike-stimulus delays between 12 and 25 ms. The increased output effects were sustained through multiple successive days of conditioning (Fig. 2A and Supplementary Fig. S2). Fig. 3 plots the sizes of the SpREs before and after conditioning in all the sessions, obtained with spike-stimulus delays between 0 and 500 ms. For delays between 12 and 25 ms the points are distributed above the unity line, indicating increases in the SpRE, for both facilitation (Fig. 3Aa) and suppression (Fig. 3Ab). These changes tended to be sustained 24 hours after the termination of conditioning (Fig. 3B), despite the resumption of normal activity. The SpREs remained larger than their original amplitudes 24 hours after the end of conditioning in 71.4 % of the cases tested (Facilitation: 16 of 22; Suppression: 4 of 6) (Fig. 2A and 3B). The SpREs for those cells tested after a second post-conditioning day are plotted individually in Fig. 3C. The SpREs remained larger than their original amplitudes 48 hours after the end of conditioning in 44.4 % of the cases tested (Facilitation: 2 of 7; Suppression: 2 of 2).
Figure 3. Summary of conditioning effect on output.
A: MPI of spike-related effects in SpTAs compiled before and after conditioning (pre MPI and post MPI). Facilitation in a; Suppression in b. Data points are color coded for different spike-stimulus delays. Data points above the dashed line indicate SpREs that increased after conditioning. The red arrow identifies session in Fig. 2A. For sessions involving multiple days of conditioning, the post-conditioning values were obtained after the last conditioning period. B: Average MPI on pre-and post-conditioning day, and one and two days after the end of conditioning, for facilitation (a) and suppression (b). Error bars show standard deviation. Significance of comparisons is given above horizontal bars. C: Individual MPI values in successive days for cell-muscle pairs that were documented for two post-conditioning days. Open circles for subsequent days indicate significant changes (p<0.05) relative to Day 0. A & b: spike-related facilitation and suppression.
Dependence of conditioning effects on delay between spikes and stimuli
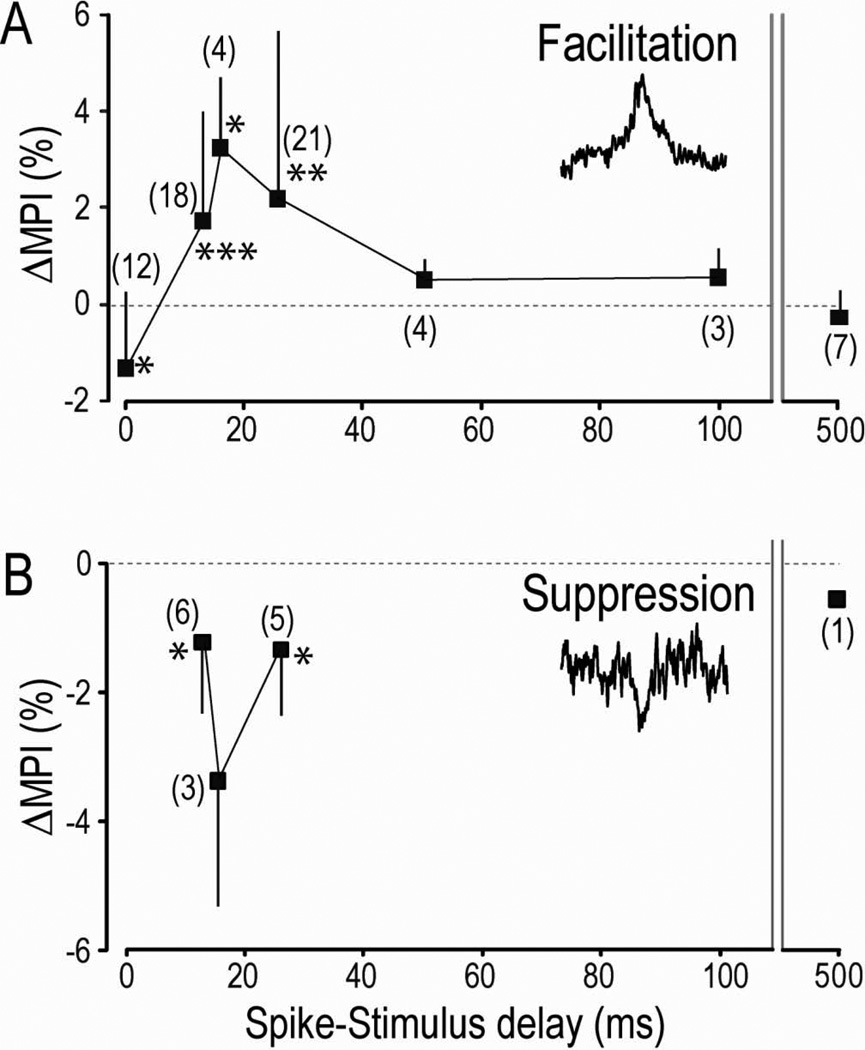
To document the dependence of the conditioning effects on the time between the CM spikes and the spinal stimuli, we performed the conditioning with different spike-stimulus delays. The overall results, plotted in Fig. 4, indicate that significant increases in amplitudes of SpREs could be produced only with delays of 12–25 ms between the action potentials and stimuli, consistent with the effective window of the standard STDP rule (Bi and Poo, 2001; Caporale and Dan, 2008; Markram et al., 2011). Delays of 50 ms and longer were ineffective in producing significant changes. Note that these latter cases involved essentially the same amount and patterns of spinal stimulation as with effective delays, so the absence of synaptic changes serves as a control for non-specific effects of stimulation.
Figure 4. Changes in spike-related effects as function of delay between spikes and stimuli.
The graphs plot average differences in MPI (ΔMPI) for pre-and post-condition days (including the unchanged cases). Numbers of separate observations are shown in parentheses. Error bars show standard deviation. Asterisks denote values significantly different from zero (* p<0.05, ** p<0.01, *** p<0.001). A: spike-related facilitation. B: spike-related suppression. For comparison, plots that include only cases in which the PSpEs changed significantly are shown in Supplementary Fig. S3.
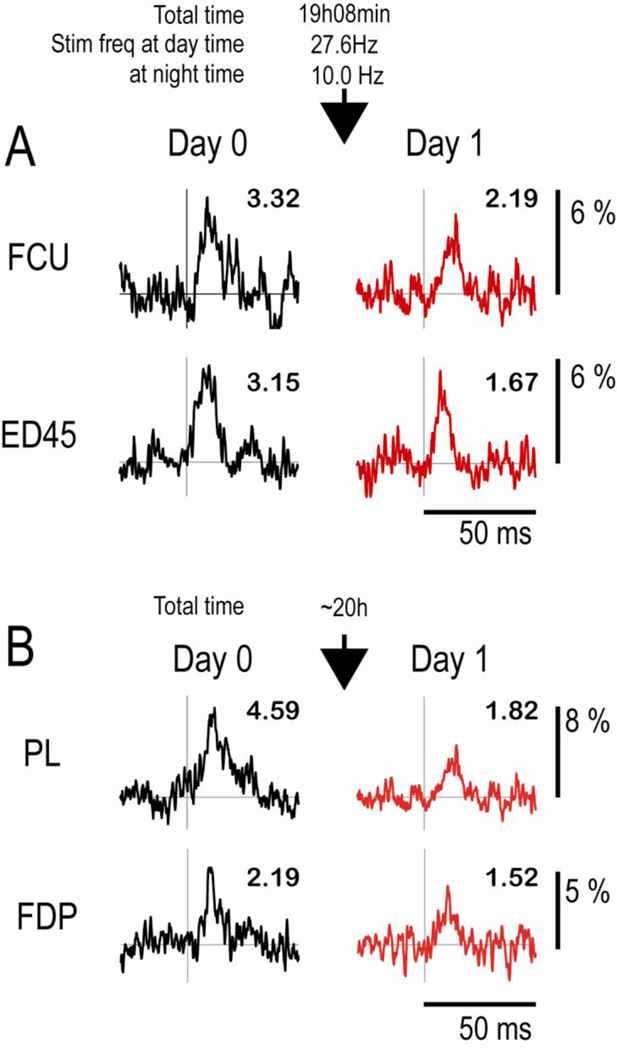
A particularly significant observation was the reduction of SpREs with zero delays between the spikes and stimuli. This average reduction in MPI was significant at the p< .05 level. Two examples, obtained in separate monkeys, are illustrated in Fig. 5. In these cases the conduction time along the corticospinal pathway caused the CM action potentials to arrive at the stimulation site after the intraspinal stimuli had activated the cells’ target motoneurons. Interestingly, two cases show a clear synchrony component prior to conditioning (ED45 in Fig. 5A and PL in Fig. 5B) that was also reduced. In these cases the earliest synchrony spikes could arrive at the spinal cord before the stimulus, but most spikes of the synchronized cells would not, because they are distributed around the trigger spikes, as shown by central peaks in cross-correlograms of CM cell pairs (Jackson et al., 2003; Smith and Fetz, 2009) (Supplementary Fig. S1B). Therefore on average the synchronized spikes would have the same net firing times relative to the spinal stimuli as the trigger spikes, and would arrive after the stimulus. The decrease in the synchrony component therefore confirms the consequent reduction of synaptic efficacy of the synchronized cell(s). The decrease in SpREs when the postsynaptic cells are activated before arrival of the presynaptic input is consistent with a bidirectional STDP rule for excitatory synapses (Caporale and Dan, 2008).
Figure 5. Decreases in SpRE produced by intraspinal stimuli with zero delay after spike.
The two cells were recorded in different monkeys (Sessions 3 & 4 in supplementary Table 1). Note the decrease in MPI values after conditioning.
Discussion
Our results provide the first demonstration that the synaptic strength of single primate CM cells can be systematically changed during unrestrained behavior. We used a recurrent brain-computer interface to trigger intraspinal stimuli from action potentials of single CM cells and activate the cells’ target motoneurons at fixed delays. This conditioning paradigm produced changes that correspond with a bidirectional STDP rule previously documented for excitatory synapses from in vitro experiments.
Neural mechanisms of conditioning effect on corticospinal connections
The synaptic efficacy of the CM cells’ corticospinal terminals was documented by the magnitude of SpREs seen in SpTAs of rectified muscle activity. The SpRE often include two distinguishable components that are superimposed (e.g., Figs. 1C, 2A and S2) and are produced by different mechanisms. A post-spike facilitation (PSpF) with an onset time consistent with serial conduction times from cortex to muscles (i.e., greater than 5 ms) is likely to be mediated by monosynaptic or disynaptic linkages from the trigger cell to motoneurons of the target muscle (Fetz and Cheney, 1980; Porter and Lemon, 1993). In addition, many SpREs show a “synchrony facilitation” evident in an increase above baseline that begins earlier and that would be produced by other CM cells that fire synchronously and have some spikes that occur prior to the trigger cell spikes (Jackson et al., 2003; Smith and Fetz, 2009; Davidson et al, 2007). Many of the SpREs show evidence of a clear PSpF superimposed on a broad synchrony peak (Figs. 2A, 5B, S1C and S2). Sometimes these two features look distinct enough to invite separation, but reliable quantification of the separate components is problematic for several reasons. The separation would require an accurate estimate of the time course of the synchrony component beneath the PSpF, which is ultimately unknown. Even if it could be reliably estimated, subtraction of the synchrony component would assume linear summation, which is not the case for averages of rectified EMG (Baker and Lemon, 1995). Moreover, in many cases these two components are hard to distinguish because the rise of the serial PSpF merges with the synchrony peak, as in Fig. S2. For these reasons we have not attempted to separate and extract quantitative measures of these superimposed components.
We found that the conditioning paradigm changed the size of the synchrony component similarly to the changes in the pure PSpF. This would be predicted from the fact that spikes of synchronized cells are distributed around the trigger cell and therefore have the same average timing relative to the spinal stimuli. Thus spike-triggered stimulation would affect the spinal terminals of other corticospinal neurons firing synchronously with the trigger cell in the same way as terminals of the trigger cell. This was seen with the increase in the size of the early synchrony component, similar to the increase in the serial PSpF (e.g., Fig. 2A, FCU, Day2, and Fig. S2; see discussion in Supplementary Materials) as well as the decrease in the synchrony component for zero delays (Fig. 5A, B).
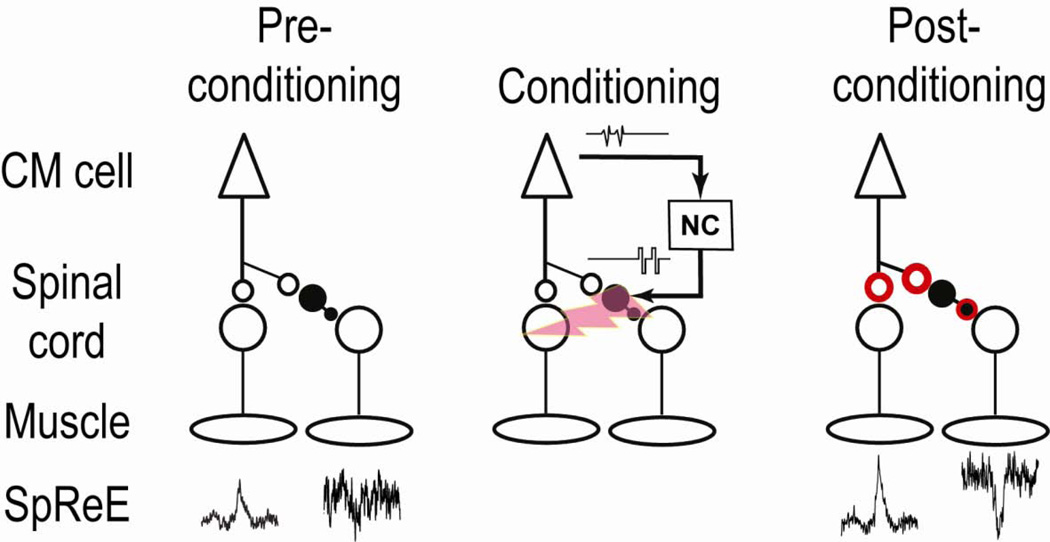
Fig. 6 illustrates the likely mechanism producing conditioned increases in the strength of corticospinal synapses and in the resultant post-spike effects for positive spike-stimulus delays. When the spinal neurons were activated 12–25 ms after arrival of the descending action potential, both excitatory and inhibitory SpREs were enhanced. Increases in the excitatory SpREs probably involve the strengthening of corticomotoneuronal terminals of the CM cells on target motoneurons, as illustrated. The mechanisms for enhancing the disynaptically mediated post-spike suppression could involve changes at two sites: in the terminals of the CM cell or terminals of the mediating inhibitory interneurons (IINs) or both. Previous studies found that the polarity of the bidirectional STDP rule is reversed for some synapses from excitatory to inhibitory neurons (Bell et al., 1997; Tzounopoulos et al., 2004). This would produce decreases in suppression, counter to our observation, but the rule for descending excitatory connections to spinal IINs remains to be determined. Given that post-spike suppression appeared in antagonists of the facilitated muscles, the underlying pathway probably involved Ia inhibitory interneurons (Jankowska et al., 1976), which are glycinergic (Fyffe, 1991). Other spinal IINs are GABAergic. In any case, assuming that stimuli activated the IINs, our evidence suggests the standard STDP rule for positive delays would apply to the corticospinal input to spinal IINs. Consistent with this, Iriki et al. found that tetanic stimulation of the pyramidal tract produced increases in corticospinal postsynaptic potentials in all of their unidentified spinal interneurons (Iriki et al., 1990). Our conditioning protocol could also strengthen the synaptic connection of the IINs to motoneurons, consistent with the STDP rule for GABAergic synapses (Haas et al., 2006; Woodin et al., 2003). Future experiments are needed to resolve the plasticity rules at these two synapses. Whatever the outcome, our results show that the net effect mediated via the disynaptic inhibitory linkage is enhanced by appropriate spike-triggered stimulation. Simultaneously strengthening both excitatory outputs to agonist muscles and inhibitory linkages to antagonists makes functional sense in maintaining the balance of effects that CM cells have on their reciprocal targets.
Figure 6. Proposed mechanisms for conditioning effect.
Spike-triggered stimulation with appropriate positive delay produces a strengthening of CM cell terminals, increasing direct excitatory post-spike effects. Changes in inhibitory effects could involve terminals of CM cell and/or inhibitory interneuron. The strengthened terminals are illustrated in red.
In vitro studies have elucidated the properties of the bidirectional STDP rules in a variety of supraspinal synapses (Bi and Poo, 2001; Caporale and Dan, 2008; Markram et al., 2011). The long corticospinal conduction time allowed demonstration that stimulation of postsynaptic cells prior to the arrival of the presynaptic impulses produced decreases in synaptic efficacy, consistent with bidirectional STDP documented in in vitro experiments. Evidence for such decreases in synaptic efficacy when postsynaptic responses precede presynaptic input has been found in sensory systems of other in vivo preparations (Jacob et al., 2007; Meliza and Dan, 2006; Mu and Poo, 2006). In most previous studies the conditioning protocol employed paired electrical stimulation. In contrast, we triggered stimuli from action potentials generated during normal behavior, which may produce more effective conditioning (Jackson et al., 2006a; Rebesco et al., 2010), perhaps due to the enhanced efficacy of natural activity patterns, known to affect plasticity (Caporale and Dan, 2008; Sjostrom et al., 2001) and possible activity-dependent delivery of facilitating neuromodulators.
Duration of conditioned effects
In most in vitro experiments the duration of so-called “long-term” potentiation and depression has typically been tested for up to several hours, and found to last that long in the absence of neural activity. In most in vivo studies the conditioned effects decay over periods of hours following the end of conditioning (Lucas and Fetz, 2013; Rebesco et al., 2010; Wolters et al., 2003). An exceptionally long-lasting effect of many days was seen with spike-triggered stimulation of nearby motor cortical sites (Jackson et al., 2006a). We found that conditioned changes in the strength of corticospinal connections lasted for variable times, in some cases up to at least two days post-conditioning. The conditioned changes of connections to different motoneuron pools from the same CM cell could survive for different durations (e.g., Fig. 2A). Thus, after the end of spike-triggered stimulation the natural activity patterns of the pre- and post-synaptic neurons tend to eventually drive synaptic strengths back to pre-conditioning levels. The synaptic strengths can be influenced by two opposing factors. Under purely random pre- and postsynaptic timings the strength of synapses would tend to decrease because the two components of the bidirectional STDP function are usually asymmetric, with greater net suppression. However, this effect is countered when presynaptic inputs tend to occur more often before the postsynaptic activity. Thus under normal behavioral conditions the balance of these and possibly other factors would determine the strength of the “steady-state” synaptic connections. Differences in the decay rate of the conditioned changes would reflect differences in the relative efficacy of these factors.
Clinical relevance
Toward promoting neural plasticity in the motor system electrical stimulation has been delivered in cerebral cortex (Brown et al., 2003) and spinal cord (Courtine et al., 2009; van den Brand et al., 2012) during movement. Such externally programmed stimulation has shown promise for facilitating recovery from the deficits of stroke (Brown et al., 2003) and spinal cord injury (Edgerton et al., 2008). The underlying mechanisms are likely to involve Hebbian strengthening of the neural connections that are activated during movements. Delivery of repetitive stimulation has typically been pre-programmed. Recent evidence suggests that a more effective strategy to promote long-lasting plasticity may be prolonged activity-dependent stimulation (Jackson et al., 2006a; Edwardson et al., 2013).
The success of our protocol suggests that neurorehabilitative treatment for patients with damaged corticospinal pathways could exploit similar paradigms to strengthen spared corticospinal connections (Jackson and Zimmerman, 2012). Previous studies have documented spinal cord conditioning produced by pairing peripheral nerve stimulation with cortical stimulation or with voluntary activation in able-bodied subjects (Khaslavskaia and Sinkjaer, 2005; Stefan et al., 2000). Pairing transcranial magnetic stimulation with antidromic activation of motoneurons at appropriate times enhanced the motor potentials evoked by stimulating corticospinal axons and improved motor performance in normal subjects (Taylor and Martin, 2009) and in patients with spinal cord injury (Bunday and Perez, 2012). A similar mechanism appears to play a role in the lasting therapeutic effects of functional electrical stimulation applied to peripheral nerves to overcome foot drop (Everaert et al., 2010).
The translation of our activity-dependent stimulation paradigm to practical clinical applications would be promoted with less invasive procedures. Instead of recording neural activity with intracortical electrodes, it is possible to record activity-dependent cortical potentials less invasively through surface electrodes (Miller et al., 2012; Nishimura et al., 2013) or to use muscle activity as a surrogate of cortical activity (Lucas and Fetz, 2013; Nishimura et al., 2013). The intraspinal stimulation that we used is known to activate many afferent fibers of passage (Gaunt et al., 2006), suggesting that less invasive spinal surface stimulation could also be effective in cortically triggered conditioning.
A second issue for clinical application is the duration of effects. In our experiments, the synaptic changes could be rapidly induced, and many eventually reverted in the days after the end of conditioning. The changes could be maintained by continuing the conditioning protocol for several days. It is possible that more prolonged conditioning could lead to more permanent changes. In future studies, longer lasting changes might also be induced by combining this paradigm with additional methods to strengthen the effects and make them more permanent (Jefferson et al., 2011; van den Brand et al., 2012). These include simultaneous DC polarization (Delgado-Lezama et al., 1999) or pharmacological intervention (Alilain et al., 2011; van den Brand et al., 2012). Another key factor in obtaining long-term effects is prolonged conditioning during volitional movements (Everaert et al., 2010), which could lead to structural changes and can be implemented with the sort of portable recurrent neural interface described here.
Experimental Procedures
Subjects
Experiments were performed with two male Macaca nemestrina monkeys (4–5 years old, weight 4.0 and 5.5 kg). All procedures were approved by the University of Washington Institutional Animal Care and Use Committee.
Recording
Activity of single CM cells in the precentral gyrus was recorded over successive days using chronically implanted, hand-moveable tungsten microwire electrodes (Jackson and Fetz, 2007) (diameter 50 µm; impedance 0.5MΩ; inter-electrode spacing 500 µm). The electrodes could be positioned along the bank of the precentral gyrus, where many CM cells reside (Rathelot and Strick, 2009; Smith and Fetz, 2009b). Each session began by recording the cell’s responses during an isometric, 2D eight-target wrist torque tracking task. Action potentials of cells were initially identified by a spike-sorting device based on a template-matching algorithm (MSD, Alpha Omega Engineering). The output effects of CM cells on forearm muscles were identified by peri-spike features in SpTAs of rectified EMG. Between these sessions of task performance, lasting about 3–5 hours, the monkey was returned to the home cage where movements were unrestricted.
Stimulation
Spinal stimuli were delivered through polyurethane-coated platinum iridium wire electrodes (diameter 30 µm; impedance 200–600kΩ) implanted chronically in the cervical spinal cord at the levels of C6-T1 (Mushahwar and Horch, 1998). Sites from which trains of stimuli evoked responses in one or more target muscles of the CM cell were chosen for conditioning. During conditioning stimulation intensities were subthreshold for generating antidromic or orthodromic responses in the recorded cells, but activated the cells’ target motoneurons.
Conditioning
The Neurochip was programmed to generate acceptance pulses when the waveform of the CM cell exceeded a threshold above the noise level and passed through two time-amplitude windows (Jackson et al., 2006; Mavoori et al., 2005). Following a specified delay after the action potential the Neurochip delivered a biphasic, constant-current stimulus pulse (0.2 ms/phase) to a wire electrode in the cervical spinal cord. To confirm unit isolation and reliable triggering during free behavior, short sections of raw recording (sampled at 11.7 kHz) were stored to on-board memory, interspersed with stimulation rate in 1 second bins during the conditioning period (Jackson et al., 2006; Mavoori et al., 2005).
Analysis
We compiled the SpTA when the cell and its target muscles were co-activated during performance of a center-out 8-target acquisition task. To facilitate comparison of SpTAs from successive days, trigger spikes were accepted for SpTAs only when they occurred within the same range of EMG activity. The numbers of triggers were equalized to the minimum number “n” obtained for any of the SpTAs by accepting only the first n triggers for all SpTAs. To de-trend the baseline from raw SpTA, the baseline trends were fitted by polynomial of appropriate order (1 to 4) and were then subtracted from the original SpTA trace. The features in the SpTAs were identified by consistent changes in values above or below 2 SD of baseline, for facilitation and suppression, respectively. Baseline was defined as the interval from 30 to 10 ms preceding the trigger spike. Onset and offset of the facilitation (or suppression) were defined as the first and last value above (or below) 2 SD of baseline. The mean percent increase (MPI) measured the average values between onset and offset of the feature minus baseline, divided by baseline (Fetz and Cheney, 1980). The MPI had negative values for suppression effects. The significance of the MPI features was determined by means of a t-test.
Significant differences in MPI produced by conditioning were determined by comparing MPI on postconditioning days with MPI on Day 0 (Fig. 1C, 2, 3C and 5), using one-way ANOVA with repeated measures. Post-hoc multiple comparisons were conducted using the Bonferroni test (Fig. 3B). To document the significance of the changes in MPI (ΔMPI, Figs. 4 and S3), we confirmed whether the mean of the distribution for each spike-stimulus delay was significantly different from zero as follows. We first tested whether the sample sets for each delay were more consistent with a normal distribution or an exponential distribution using the Lilliefors test (p>0.05). For the normally distributed values the single-tailed Student t-test was used to determine whether the mean of the distribution was significantly different from zero. For the exponentially distributed values the Wilcoxen signed-rank test was used to determine whether the distribution median was significantly different from zero. The p values for differences from zero are designated by asterisks. For points designated by “(1)” the number of samples was too small to provide meaningful statistics.
Supplementary Material
Highlights.
-
-
Activity-dependent stimuli in spinal cord induced STDP in corticospinal terminals
-
-
Bidirectional STDP was documented at the single neuron level
-
-
A head-fixed recurrent neural interface produced STDP during free behavior
Acknowledgements
We thank A. Price and R. Robinson for technical help and L. Shupe for programming. The work was supported by grants from the National Institutes of Health NS 12542, Christopher & Dana Reeve Foundation, Life Sciences Discovery Fund, the W.M. Keck Foundation for E.F., the National Institutes of Health NS 40867 for S.I.P and Precursory Research for Embryonic Science and Technology, Japan Science and Technology Agency for Y.N.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Supplementary Materials
Components of post-spike effects
Synchrony facilitation is modulated like post-spike facilitation
Figure S1
Figure S2
Figure S3
Table S1
References in Supplementary materials
Contributions
Y.N. and R.W.E. conducted the experiments. Y.N. and R.W.E. analyzed the data. E.E.F., Y.N. and S.I.P. conceived the study and wrote the manuscript.
Competing financial interests
The authors declare no competing financial interests.
References
- Alilain WJ, Horn KP, Hu H, Dick TE, Silver J. Functional regeneration of respiratory pathways after spinal cord injury. Nature. 2011;475:196–200. doi: 10.1038/nature10199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker SN, Lemon RN. Non-linear summation of responses in averages of rectified EMG. J Neurosci Methods. 1995;59:175–181. doi: 10.1016/0165-0270(94)00180-o. [DOI] [PubMed] [Google Scholar]
- Bell CC, Han VZ, Sugawara Y, Grant K. Synaptic plasticity in a cerebellum-like structure depends on temporal order. Nature. 1997;387:278–281. doi: 10.1038/387278a0. [DOI] [PubMed] [Google Scholar]
- Bennett KM, Lemon RN. The influence of single monkey cortico-motoneuronal cells at different levels of activity in target muscles. Journal of Physiology. 1994;477:291–307. doi: 10.1113/jphysiol.1994.sp020191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bi G, Poo M. Synaptic modification by correlated activity: Hebb's postulate revisited. Annual review of neuroscience. 2001;24:139–166. doi: 10.1146/annurev.neuro.24.1.139. [DOI] [PubMed] [Google Scholar]
- Brown JA, Lutsep H, Cramer SC, Weinand M. Motor cortex stimulation for enhancement of recovery after stroke: case report. Neurol Res. 2003;25:815–818. doi: 10.1179/016164103771953907. [DOI] [PubMed] [Google Scholar]
- Bunday KL, Perez MA. Motor recovery after spinal cord injury enhanced by strengthening corticospinal synaptic transmission. Curr Biol. 2012;22:2355–2361. doi: 10.1016/j.cub.2012.10.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caporale N, Dan Y. Spike timing-dependent plasticity: a Hebbian learning rule. Annual review of neuroscience. 2008;31:25–46. doi: 10.1146/annurev.neuro.31.060407.125639. [DOI] [PubMed] [Google Scholar]
- Courtine G, Gerasimenko Y, van den Brand R, Yew A, Musienko P, Zhong H, Song B, Ao Y, Ichiyama RM, Lavrov I, et al. Transformation of nonfunctional spinal circuits into functional states after the loss of brain input. Nat Neurosci. 2009;12:1333–1342. doi: 10.1038/nn.2401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cramer SC, Sur M, Dobkin BH, O'Brien C, Sanger TD, Trojanowski JQ, Rumsey JM, Hicks R, Cameron J, Chen D, et al. Harnessing neuroplasticity for clinical applications. Brain. 2011;134:1591–1609. doi: 10.1093/brain/awr039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delgado-Lezama R, Perrier JF, Hounsgaard J. Local facilitation of plateau potentials in dendrites of turtle motoneurones by synaptic activation of metabotropic receptors. J Physiol. 1999;515(Pt 1):203–207. doi: 10.1111/j.1469-7793.1999.203ad.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davidson AG, Chan V, O'Dell R, Schieber MH. Rapid changes in throughput from single motor cortex neurons to muscle activity. Science. 2007;318:1934–1937. doi: 10.1126/science.1149774. [DOI] [PubMed] [Google Scholar]
- Edgerton VR, Courtine G, Gerasimenko YP, Lavrov I, Ichiyama RM, Fong AJ, Cai LL, Otoshi CK, Tillakaratne NJ, Burdick JW, Roy RR. Training locomotor networks. Brain Res Rev. 2008;57:241–254. doi: 10.1016/j.brainresrev.2007.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edwardson M, Lucas T, Carey J, Fetz E. New modalities of brain stimulation for stroke rehabilitation. Experimental Brain Research. 2013;224:335–358. doi: 10.1007/s00221-012-3315-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Everaert DG, Thompson AK, Chong SL, Stein RB. Does functional electrical stimulation for foot drop strengthen corticospinal connections? Neurorehabil Neural Repair. 2010;24:168–177. doi: 10.1177/1545968309349939. [DOI] [PubMed] [Google Scholar]
- Fetz EE, Cheney PD. Postspike facilitation of forelimb muscle activity by primate corticomotoneuronal cells. J Neurophysiol. 1980;44:751–772. doi: 10.1152/jn.1980.44.4.751. [DOI] [PubMed] [Google Scholar]
- Froemke RC, Debanne D, Bi GQ. Temporal modulation of spike-timing-dependent plasticity. Front Synaptic Neurosci. 2010;2:19. doi: 10.3389/fnsyn.2010.00019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fyffe RE. Glycine-like immunoreactivity in synaptic boutons of identified inhibitory interneurons in the mammalian spinal cord. Brain Res. 1991;547:175–179. doi: 10.1016/0006-8993(91)90590-r. [DOI] [PubMed] [Google Scholar]
- Gaunt RA, Prochazka A, Mushahwar VK, Guevremont L, Ellaway PH. Intraspinal microstimulation excites multisegmental sensory afferents at lower stimulus levels than local alpha-motoneuron responses. J Neurophysiol. 2006;96:2995–3005. doi: 10.1152/jn.00061.2006. [DOI] [PubMed] [Google Scholar]
- Haas JS, Nowotny T, Abarbanel HD. Spike-timing-dependent plasticity of inhibitory synapses in the entorhinal cortex. J Neurophysiol. 2006;96:3305–3313. doi: 10.1152/jn.00551.2006. [DOI] [PubMed] [Google Scholar]
- Iriki A, Keller A, Pavlides C, Asanuma H. Long-lasting facilitation of pyramidal tract input to spinal interneurons. Neuroreport. 1990;1:157–160. [PubMed] [Google Scholar]
- Jackson A, Fetz EE. Compact movable microwire array for long-term chronic unit recording in cerebral cortex of primates. J Neurophysiol. 2007;98:3109–3118. doi: 10.1152/jn.00569.2007. [DOI] [PubMed] [Google Scholar]
- Jackson A, Gee VJ, Baker SN, Lemon RN. Synchrony between neurons with similar muscle fields in monkey motor cortex. Neuron. 2003;38:115–125. doi: 10.1016/s0896-6273(03)00162-4. [DOI] [PubMed] [Google Scholar]
- Jackson A, Mavoori J, Fetz EE. Long-term motor cortex plasticity induced by an electronic neural implant. Nature. 2006a;444:56–60. doi: 10.1038/nature05226. [DOI] [PubMed] [Google Scholar]
- Jackson A, Mavoori J, Fetz EE. Correlations between the same motor cortex cells and arm muscles during a trained task, free behavior, and natural sleep in the macaque monkey. J Neurophysiol. 2007;97:360–374. doi: 10.1152/jn.00710.2006. [DOI] [PubMed] [Google Scholar]
- Jackson A, Moritz CT, Mavoori J, Lucas TH, Fetz EE. The Neurochip BCI: towards a neural prosthesis for upper limb function. IEEE Trans Neural Syst Rehabil Eng. 2006b;14:187–190. doi: 10.1109/TNSRE.2006.875547. [DOI] [PubMed] [Google Scholar]
- Jackson A, Zimmerman JB. Neural interfaces for the brain and spinal cord – restoring motor function, Nat. Rev. Neurol. 2012;8:690–699. doi: 10.1038/nrneurol.2012.219. [DOI] [PubMed] [Google Scholar]
- Jacob V, Brasier DJ, Erchova I, Feldman D, Shulz DE. Spike timing-dependent synaptic depression in the in vivo barrel cortex of the rat. J Neurosci. 2007;27:1271–1284. doi: 10.1523/JNEUROSCI.4264-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jankowska E, Padel Y, Tanaka R. Disynaptic inhibition of spinal motoneurones from the motor cortex in the monkey. J Physiol. 1976;258:467–487. doi: 10.1113/jphysiol.1976.sp011431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jefferson SC, Tester NJ, Howland DR. Chondroitinase ABC promotes recovery of adaptive limb movements and enhances axonal growth caudal to a spinal hemisection. J Neurosci. 31:5710–5720. doi: 10.1523/JNEUROSCI.4459-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khaslavskaia S, Sinkjaer T. Motor cortex excitability following repetitive electrical stimulation of the common peroneal nerve depends on the voluntary drive. Exp Brain Res. 2005;162:497–502. doi: 10.1007/s00221-004-2153-1. [DOI] [PubMed] [Google Scholar]
- Kleim JA. Neural Plasticity: Implications for Rehabilitation. 1 edn. Plural Publishing Inc; 2008. [DOI] [PubMed] [Google Scholar]
- Lucas TH, Fetz EE. Myo-cortical crossed feedback reorganizes primate motor cortex output. J Neurosci. 2013;33:5261–5274. doi: 10.1523/JNEUROSCI.4683-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Markram H, Gerstner W, Sjostrom PJ. A history of spike-timing-dependent plasticity. Front Synaptic Neurosci. 2011 doi: 10.3389/fnsyn.2011.00004. 2011;3:4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meliza CD, Dan Y. Receptive-field modification in rat visual cortex induced by paired visual stimulation and single-cell spiking. Neuron. 2006;49:183–189. doi: 10.1016/j.neuron.2005.12.009. [DOI] [PubMed] [Google Scholar]
- Miller KJ, Hermes D, Honey CJ, Hebb AO, Ramsey NF, Knight RT, Ojemann JG, Fetz EE. Human motor cortical activity is selectively phase-entrained on underlying rhythms. PLoS Comput Biol. 2012;8:e1002655. doi: 10.1371/journal.pcbi.1002655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mu Y, Poo MM. Spike timing-dependent LTP/LTD mediates visual experience-dependent plasticity in a developing retinotectal system. Neuron. 2006;50:115–125. doi: 10.1016/j.neuron.2006.03.009. [DOI] [PubMed] [Google Scholar]
- Mushahwar VK, Horch KW. Selective activation and graded recruitment of functional muscle groups through spinal cord stimulation. Ann N Y Acad Sci. 1998;860:531–535. doi: 10.1111/j.1749-6632.1998.tb09096.x. [DOI] [PubMed] [Google Scholar]
- Nishimura Y, Perlmutter SI, Fetz EE. Restoration of upper limb movement via artificial corticospinal and musculospinal connections in a monkey with spinal cord injury. Frontiers in neural circuits. 2013;7:57. doi: 10.3389/fncir.2013.00057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Porter R, Lemon RN. Corticospinal Function and Voluntary Movement. Oxford: Oxford Univ. Press; 1993. [Google Scholar]
- Rathelot JA, Strick PL. Muscle representation in the macaque motor cortex: an anatomical perspective. Proc Natl Acad Sci U S A. 2006;103:8257–8262. doi: 10.1073/pnas.0602933103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rebesco JM, Stevenson IH, Kording KP, Solla SA, Miller LE. Rewiring neural interactions by micro-stimulation. Front Syst Neurosci. 2010;4 doi: 10.3389/fnsys.2010.00039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salgado H, Kohr G, Trevino M. Noradrenergic 'tone' determines dichotomous control of cortical spike-timing-dependent plasticity. Sci Rep. 2:417. doi: 10.1038/srep00417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schieber MH, Rivlis G. A spectrum from pure post-spike effects to synchrony effects in spike-triggered averages of electromyographic activity during skilled finger movements. J Neurophysiol. 2005;94:3325–3341. doi: 10.1152/jn.00007.2005. [DOI] [PubMed] [Google Scholar]
- Seol GH, Ziburkus J, Huang S, Song L, Kim IT, Takamiya K, Huganir RL, Lee HK, Kirkwood A. Neuromodulators control the polarity of spike-timing-dependent synaptic plasticity. Neuron. 2007;55:919–929. doi: 10.1016/j.neuron.2007.08.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sjostrom PJ, Turrigiano GG, Nelson SB. Rate, timing, and cooperativity jointly determine cortical synaptic plasticity. Neuron. 2001;32:1149–1164. doi: 10.1016/s0896-6273(01)00542-6. [DOI] [PubMed] [Google Scholar]
- Smith WS, Fetz EE. Synaptic linkages between corticomotoneuronal cells affecting forelimb muscles in behaving primates. J Neurophysiol. 2009;102:1040–1048. doi: 10.1152/jn.91052.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stefan K, Kunesch E, Cohen LG, Benecke R, Classen J. Induction of plasticity in the human motor cortex by paired associative stimulation. Brain. 2000;123(Pt 3):572–584. doi: 10.1093/brain/123.3.572. [DOI] [PubMed] [Google Scholar]
- Taylor JL, Martin PG. Voluntary motor output is altered by spike-timing-dependent changes in the human corticospinal pathway. J Neurosci. 2009;29:11708–11716. doi: 10.1523/JNEUROSCI.2217-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tzounopoulos T, Kim Y, Oertel D, Trussell LO. Cell-specific, spike timing-dependent plasticities in the dorsal cochlear nucleus. Nat Neurosci. 2004;7:719–725. doi: 10.1038/nn1272. [DOI] [PubMed] [Google Scholar]
- van den Brand R, Heutschi J, Barraud Q, DiGiovanna J, Bartholdi K, Huerlimann M, Friedli L, Vollenweider I, Moraud EM, Duis S, et al. Restoring voluntary control of locomotion after paralyzing spinal cord injury. Science. 2012;336:1182–1185. doi: 10.1126/science.1217416. [DOI] [PubMed] [Google Scholar]
- Wolpaw JR, Tennissen AM. Activity-dependent spinal cord plasticity in health and disease. Annual review of neuroscience. 2001;24:807–843. doi: 10.1146/annurev.neuro.24.1.807. [DOI] [PubMed] [Google Scholar]
- Wolters A, Sandbrink F, Schlottmann A, Kunesch E, Stefan K, Cohen LG, Benecke R, Classen J. A temporally asymmetric Hebbian rule governing plasticity in the human motor cortex. J Neurophysiol. 2003;89:2339–2345. doi: 10.1152/jn.00900.2002. [DOI] [PubMed] [Google Scholar]
- Woodin MA, Ganguly K, Poo MM. Coincident pre-and postsynaptic activity modifies GABAergic synapses by postsynaptic changes in Cl-transporter activity. Neuron. 2003;39:807–820. doi: 10.1016/s0896-6273(03)00507-5. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.