Abstract
During myocardial ischemia, upregulation of the hedgehog (Hh) pathway promotes neovascularization and increases cardiomyocyte survival. The canonical Hh pathway activates a transcriptional program through the Gli family of transcription factors by derepression of the seven-transmembrane protein smoothened (Smo). The mechanisms linking Smo to Gli are complex and, in some cell types, involve coupling of Smo to Gi proteins. In the present study, we investigated, for the first time, the transcriptional response of cardiomyocytes to sonic hedgehog (Shh) and the role of Gi protein utilization. Our results show that Shh strongly activates Gli1 expression by quantitative PCR in a Smo-dependent manner in neonatal rat ventricular cardiomyocytes. Microarray analysis of gene expression changes elicited by Shh and sensitive to a Smo inhibitor identified a small subset of 37 cardiomyocyte-specific genes regulated by Shh, including some in the PKA and purinergic signaling pathways. In addition, neonatal rat ventricular cardiomyocytes infected with an adenovirus encoding GiCT, a peptide that impairs receptor-Gi protein coupling, showed reduced activation of Hh targets. In vitro data were confirmed in transgenic mice with cardiomyocyte-inducible GiCT expression. Transgenic GiCT mice showed specific reduction of Gli1 expression in the heart under basal conditions and failed to upregulate the Hh pathway upon ischemia and reperfusion injury, unlike their littermate controls. This study characterizes, for the first time, the transcriptional response of cardiomyocytes to Shh and establishes a critical role for Smo coupling to Gi in Hh signaling in the normal and ischemic myocardium.
Keywords: hedgehog, smoothened, G proteins, heart failure
the critical role of the hedgehog (Hh) signaling pathway in tissue homeostasis, stem cell renewal, and angiogenesis has triggered a growing interest in its utilization for regenerative medicine. The Hh pathway is initiated by any of three secreted family members [sonic hedgehog (Shh), indian hedgehog, and desert hedgehog], with Shh being the most relevant in the myocardium. In the absence of Shh, the 12-transmembrane receptor patched1 (Ptc1) represses smoothened (Smo), a 7-transmembrane protein that regulates activation of the Gli family of transcription factors (27). In the presence of Shh, Ptc1 is internalized and Smo is derepressed, traffics to the membrane of the primary cilium, and allows activation of constitutively expressed Gli2 and Gli3 isoforms, which leads to the induction of Gli-target genes, including Gli1 (the most sensitive Hh target gene and most active Gli isoform). This is the so-called “canonical” or transcriptional Hh pathway (4). We (26) recently demonstrated that Smo can function as a G protein-coupled receptor (GPCR) with strong selectively toward all members of the Gi protein family. Activation of Gi by Smo is necessary for Gli activation in some but not all cell types, likely due to differential basal levels of PKA activity. PKA is a strong negative regulator of canonical Hh signaling via phosphorylation of Gli2 and Gli3, which primes them for additional modification by glycogen synthase-3β and casein kinase-1 and subsequent partial proteasomal degradation (24, 25, 31). Gi proteins, however, play a central role in mediating noncanonical Hh signaling, which is independent of Gli transcription and results in rapid changes in cell morphology, migration, or Ca2+ uptake (2, 6, 22, 23).
Shh plays an essential role during embryonic development of the vertebrate heart and in myocardial regeneration in the zebrafish. This organism is able to regenerate the myocardium after a small resection of the ventricular vertex. Interestingly, administration of a Smo agonist increased cardiomyocyte proliferation, and a Smo inhibitor reduced proliferation and regeneration postinjury (7). In mammals, Shh exerts a critical role during heart development by promoting cardiomyoblast proliferation and angiogenesis during secondary heart field development (9, 10, 16).
In the adult mammalian myocardium, Shh is expressed by interstitial and adventitial fibroblasts and induces classical Hh target genes in the surrounding fibroblasts, perivascular cells, and, less clearly, cardiomyocytes (15). Shh stimulates angiogenesis in vivo and in vitro. The mechanism appears to involve a combinatorial effect of upregulation of proangiogenic factors by tissue fibroblasts in a Gli-dependent manner and a direct effect on endothelial cell tubulogenesis and survival independently of Gli transcriptional activity, through a noncanonical pathway (6, 21). We (6) have shown that endothelial cells respond to Hh ligands with extensive cytoskeletal changes that are mediated by Smo acting as a GPCR through Gi proteins and by activation of Rac1 and RhoA. A distinguishable function of Hh ligands serves to reduce endothelial cell apoptosis by serum withdrawal by inhibition of a proapoptotic function of Ptc1 (6). It is likely that Smo participates in vascular homeostasis of the heart, as suggested by a study (15) in which acute deletion of Smo with a floxed allele resulted in vasculature collapse and heart failure. However, there is no evidence of vascular density loss in animals and humans treated with different Smo inhibitors for prolonged periods of time, suggesting that perhaps Smo has an unknown function in the vasculature independent of Hh signal transduction.
The role of Hh signaling in the diseased heart is more controversial. During tissue hypoxia, Shh is upregulated and contributes to boost the capillary density and to restore adequate perfusion in both the myocardium and skeletal muscle (14, 20). Supporting Hh signaling in the ischemic heart by intramyocardial delivery of a Shh-encoding plasmid resulted in a significant increase in angiogenesis and reduced fibrosis (14). Moreover, upregulation of Shh in cardiomyocytes was proposed to mediate the cardioprotective effect of erythropoietin, through the induction of VEGF and angiopoietin-1, and to promote neovascularization reducing infarct size and alleviating cardiac dysfunction (30). Stem cell therapy in infarcted rat hearts with mesenchymal stem cells engineered to overexpress Shh resulted in increased survival of engrafted stem cells and enhanced angiogenesis (1). Thus, ample evidence demonstrates the proangiogenic role of Shh after myocardial infarction and after ischemia and reperfusion injury. However, another study (3) reported that blockade of endogenous Hh signaling with the drug cyclopamine (a Smo inhibitor) in a mouse model of cardiac ischemia and reperfusion ameliorated ventricular dilation and improved cardiac output, mainly through reduction of the fibrotic response. In addition to this controversy regarding the cardioprotective or cardiotoxic role of endogenous Hh signaling after ischemia, evidence for the role of Hh signaling in cardiomyocytes, not in mesenchymal and vascular cells, is lacking.
Given the paucity of information regarding the existence, features, and function of Hh signaling in cardiac myocytes, we investigated the capacity of neonatal cardiomyocytes to activate the canonical Hh signaling pathway in vitro and the requirement of Gi proteins and characterized the most responsive cell type-specific genes. In addition, we studied the role of Gi in the induction of Gli target genes in the whole heart after ischemia-reperfusion (I/R) injury using a transgenic mouse model with cardiomyocyte-specific expression of GiCT, a peptide that prevents Gi protein activation by GPCRs. Our results formally demonstrate that cardiomyocytes respond to Shh in vitro and in vivo and exhibit a classical canonical activation of Gli target genes in a Gi protein-dependent manner. Induction of the Hh pathway after I/R injury is blunted in the absence of Gi protein signaling and correlates with an increase in infarct size, demonstrating the physiological significance of Hh signaling in the myocardium proper.
MATERIALS AND METHODS
Antibodies.
The following antibodies were purchased from Cell Signaling Technology (Danvers, MA): anti-hemagglutinin (HA; C29F4) and anti-Gli1 (L42B10). Antibodies directed to alkaline phosphatase (Alpl) and ceruloplasmin (Cp) were from GenTex (Irvine, CA). Secondary antibodies conjugated to horseradish peroxidase were from Bio-Rad (Hercules, CA).
Animals.
Sprague-Dawley rat pups (postnatal days 1–3) for the isolation of neonatal rat ventricular myocytes (NVRMs) were obtained from S-A Ace (Boyertown, PA). Transgenic cardiomyocyte-specific inducible GiCT mice (GiCT/TTA) were obtained by breeding mice encoding a doxycyline-sensitive TTA transgene [α-myosin heavy chain (MHC)-TTA] with mice encoding a TTA-driven α-MHC-GiCT transgene, as previously described (8). None of the transgenic animals showed any overt phenotype under nonstress conditions, but GiCT/TTA mice did have a reduced recovery of cardiac function after I/R injury (8). For cardiomyocyte GiCT silencing, doxycycline was administered at 300 mg/kg of mouse diet (Bio-Serv, Frenchtown, NJ) for at least 2 wk.
Animals were housed, handled, and euthanized according to the National Institutes of Health Guide for the Care and Use of Laboratory Animals. All procedures were approved by the Thomas Jefferson University Committee on Animal Care.
Adenoviruses.
The recombinant adenovirus encoding HA-tagged GiCT has been previously described (8). Control LacZ adenovirus was obtained from Agilent Technologies (Santa Clara, CA). Both adenoviruses were amplified in AD-293 cells, and titers were determined using the AdEasy Viral Titer kit (Agilent). Both adenoviruses were used at titers of >108 plaque-forming units/ml.
Purification of recombinant Shh.
Recombinant Shh was synthesized and purified as previously described (18). Briefly, the NH2-terminal fragment of Shh was expressed in BL21 bacteria fused to NH2-terminal calmodulin-binding peptide (CBP). CBP-Shh protein was purified by affinity chromatography with calmodulin-sepharose in the presence of Ca2+ and eluted by an excess of EGTA. The CBP tag was then cleaved by enterokinase, and both CBP and enterokinase were removed by affinity chromatography. NH2-Shh was concentrated and cleaned of lipopolysaccharides with a Detoxi-Gel Endotoxin removal resin (Pierce). The activity and concentration of NH2-Shh were determined by SDS-PAGE stained with Coomassie blue and by Gli-luciferase assay in NIH-3T3 cells, as previously described (18).
Isolation and culture of primary NRVMs with Hh signaling modulators.
Primary NRVMs were isolated from 1- to 2-day-old Sprague-Dawley rat hearts. Briefly, the ventricles of neonatal hearts were minced in 116 mmol/l NaCl, 20 mmol/l HEPES (pH 7.35), 0.8 mmol/l Na2HPO4, 5.6 mmol/l glucose, 5.4 mmol/l KCl, and 0.8 mmol/l MgSO4 containing 0.6 g/l pancreatin (Sigma, St. Louis, MO) and 0.4 g/l collagenase type II (Worthington, Lakewood, NJ) in a rotating water bath at 37°C followed by protease inactivation with FBS (Life Technologies, Grand Island, NY) and centrifuged for 5 min at 100 g. The pellet was resuspended in Ham's F-10 medium (Mediatech, Manassas, VA) containing 10% horse serum (GIBCO-Life Technologies), 5% FBS, 100 μg/l penicillin-streptomycin (Mediatech), and 100 μmol/l bromodeoxyuridine (Fisher Scientific). The suspension was then passed through a 70-μm nylon filter and plated for 90 min to remove cardiac fibroblasts by selective adhesion. NRVMs were then counted in trypan blue I (Mediatech) and seeded at 0.5 × 106 cells/ml in Falcon Primaria tissue culture plates (Becton Dickinson, Franklin Lakes, NJ). When adenoviruses were used, NRVMs were infected with AdV-GiCT or AdV-LacZ on day 2 after isolation. After 24 h of infection, media were replaced by Ham's F-10 with 0.5% FBS and 100 μg/l penicillin-streptomycin. NRVMs were then incubated 2 h in the presence of 0.5 μmol/l KAAD-cyclopamine (EMD Millipore, Billerica, MA) or DMSO vehicle followed by 24-h treatment with 5 μmol/l purmorphamine (EMD Millipore), 2.5 mg/l recombinant Shh, or vehicle.
Quantitative real-time PCR.
Total RNA was extracted from flash-frozen hearts or brains of 8- to 10-wk-old GiCT/TTA and control TTA mice by guanidinium thiocyanate-phenol-chloroform extraction (Ultraspec RNA Isolation System, BIOCTEX, Houston, TX) according to the manufacturer's instructions and treated with DNase I (TURBO DNase, Life Technologies) for 30 min. cDNA was synthesized using the SuperScript First-Strand Synthesis System (Invitrogen-Life Technologies) from 1 μg total RNA using oligo (dT) primers. Total RNA from cultured NRVMs was isolated using the RNeasy Mini Kit (Qiagen). DNase I-treated NRVM cDNA was synthesized using the High-Capacity cDNA Reverse Transcription Kit (Applied Biosystems, Life Technologies) with hexarandom primers.
Quantitative real-time PCR was performed in a Mini Opticon MJ Mini Thermal Cycler (Bio-Rad) using SsoFast EvaGreen qPCR Supermix reagent (Bio-Rad). The following primers were used: rat Gli1, forward 5′-CACGTGCAACCTGCCAGCTGA-3′ and reverse 5′-GCATCCCCAGCAGGTGATCCTGT-3′; mouse Gli1, forward 5′-GGACTTTCTGGTCTGCCCTTTTG-3′ and reverse 5′-ATGGAGAGAGCCCGCTTCTTTG-3′; rat RNA polymerase II (Pol2), forward 5′-CCTGGAGACAGATGGTGTGAG-3′ and reverse 5′-AATGCCCAGTACCGTGAAG-3′; and mouse ribosomal protein S15 (S15), forward 5′-TTCCGCAAGTTCACCTACC-3′ and reverse 5′-CGGGCCGGCCGTGCTTCACG-3′; housekeeping genes Pol2 and S15 were used for normalization. Results were analyzed by the 2−ΔΔCt (where Ct is threshold cycle) method using Bio-Rad CFX Manager 1.6 software (Bio-Rad). Experiments were repeated three or four times in triplicate.
Microarray analysis.
RNA extracted from cultured NRVMs was reverse transcribed, labeled, and hybridized to rat gene 1.0 ST arrays (Affymetrix, Santa Clara, CA). Chips were scanned on an Affymetrix Gene Chip Scanner 3000 using Command Console software. Background correction and normalization were done using Iterative Plier 16 with GeneSpring (version 12.0) software (Agilent). A 1.5-fold (P < 0.05) differentially expressed gene list was generated. The differentially expressed gene list was loaded into Ingenuity Pathway Analysis 8.0 software (http://www.ingenuity.com) to perform biological network and functional analyses.
I/R injury model.
Mice under isoflurane anesthesia were subjected to a thoracotomy and ligation of the left anterior descending coronary artery (LAD) using a slipknot, as previously described (11). Sham-operated (sham) animals were subjected to the same surgical procedures except that the suture was passed under the LAD but was not tied. After 30 min of ischemia, the slipknot in the I/R group was released, and the myocardium was reperfused. Animals received a single dose of buprenorphine (0.05 mg/kg sc) immediately after reperfusion for postoperative analgesia. Hearts were harvested after 72 h for total RNA extraction.
Immunohistochemistry.
Analysis of expression of GiCT in the myocardium of 8- to 10-wk-old animals was carried out by immunohistochemistry using anti-HA (C29F4) rabbit monoclonal antibody (Cell Signaling Technology) and the goat anti-rabbit EnVision System (horseradish peroxidase-labeled polymer, Dako) for staining.
Intracellular cAMP determination.
NRVMs were plated at a concentration of 1 × 105 cells/well in standard 96-well microplates, transduced with AdV-lacZ or AdV-GiCT, and cultured overnight. Cells were stimulated the following day for 5 min with 10 μmol/l isoproterenol (Iso) or vehicle in triplicate. Some wells were treated with 5 μM purmorphamine or with 2.5 mg/l recombinant Shh for 30 min at 37°C before stimulation with Iso. Application of 0.5 μmol/l KAAD-cyclopamine to some wells was done 3 h before the addition of purmorphamine or Shh. Intracellular cAMP was extracted and assayed by enzyme immunoassay according to the manufacturer's instructions (Biotrak, GE Health Care Biosciences, Pittsburgh, PA). Absorbance values were transformed to cAMP concentrations with use of a standard curve generated by plotting the percent bound for each standard and sample as a function of the log cAMP concentration.
RESULTS
Shh activates a canonical pathway in isolated NRVMs.
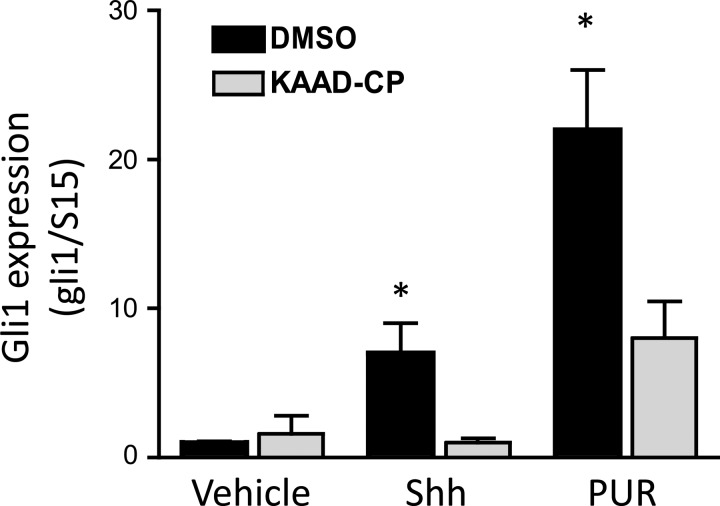
NRVMs were isolated and cultured for 24 h followed by the addition of 2.5 mg/l recombinant Shh. After 24 h of incubation, cells were lysed, and total RNA was extracted for cDNA synthesis and quantitative PCR of canonical Gli target genes. As shown in Fig. 1, Shh increased the expression level of Gli1, a highly responsive Hh gene, by approximately sevenfold. Coincubation of cells with 0.5 μmol/l KAAD-cyclopamine, a specific inhibitor of Smo, abolished the induction of Gli1 by Shh. This result suggests that the canonical Hh pathway is intact in cardiomyocytes and that the central signal transducer Smo is a required mediator of Gli activation. To further prove the latter, application of purmorphamine (5 μmol/l), a direct Smo agonist (29), was sufficient to strongly stimulate Gli1 expression (>20-fold) and was also efficiently reduced by 0.5 μmol/l KAAD-cyclopamine, which competes with purmorphamine for binding to Smo. These observations indicate that ventricular cardiomyocytes are Hh-responsive cells.
Fig. 1.
Canonical hedgehog (Hh) signaling in cardiomyocytes. Neonatal rat ventricular myocytes (NRVMs) were cultured as described in materials and methods for 24 h and then switched to low serum medium supplemented with 2.5 mg/l sonic hedgehog (Shh) or 5 μmol/l purmorphamine (PUR) alone or in the presence of 5 μmol/l KAAD-cyclopamine (KAAD-CP). After 24 h, total RNA was isolated and processed for quantitative RT-PCR of Gli1 and the housekeeping gene ribosomal protein S15 (S15). Results are means ± SE of the normalized ratio of Gli1 to S15 from 3 independent experiments; n = 4. *P < 0.025.
Microarray analysis reveals a cardiomyocyte-specific set of Hh-responsive genes.
Subsequently, we sought to examine the cell type-specific regulation of Hh target genes by analyzing global genome changes by microarray. Total cDNA generated from NRVMs treated during 24 h with vehicle, Shh, or Shh plus KAAD-cyclopamine was hybridized to Affymetrix rat gene chips for the quantification of relative expression levels. Results from two independent experiments were compared, and only those genes that were specifically up- or downregulated in the Shh group but not in the presence of the Smo inhibitor KAAD-cyclopamine were considered for further analysis. Using this condition, there were 29 genes specifically upregulated and 8 genes specifically downregulated by Shh. A heat map of the duplicate experiments containing the 37 genes with significant changes is shown in Fig. 2A. Pathway analysis revealed that among the most represented signaling pathways were Shh, PKA, transforming growth factor-β, fibrosis, β-adrenergic signaling, and retinoic acid. A selected group of upregulated genes was further validated by quantitative PCR analysis (Fig. 2B). The data showed some upregulated genes that are prototypical Gli-responsive genes (Gli1, Ptch1, and Alpl) and some strongly upregulated genes that have not been previously reported as responsive to Hh signaling [Cp, arylsulfatase, retinol dehydrogenase 10, ectonucleoside triphosphate diphosphohydrolase 2 (Endtp2), A-kinase anchor protein 12, a predicted inhibitory lectin (loc689757), IL-13 receptor α2-subunit, endocan, podocalyxin, and others]. The latter represent a novel subset of cardiomyocyte Hh-responsive genes. Western blot analysis of selected target genes confirmed that Gli1, Alpl, and Cp are upregulated at the protein level (Fig. 2C).
Fig. 2.
Identification of Shh-responsive genes in cardiomyocytes. A: microarray heat map of the subset of genes specifically modulated by Shh in NRVMs cultured 24 h with vehicle or 2.5 μg/l Shh with or without 0.5 μmol/l KAAD-CP. The first, third, and fifth columns are raw expression values from experiment 1, whereas the second, fourth, and sixth columns are raw values from experiment 2. Color code: bright green < dark green < black < dark red < bright red. B: quantitative RT-PCR validation of selected genes from the microarray analysis. Values are means ± SE of induction (Shh/vehicle) of 3 independent experiments and were calculated by the ΔΔCt (where Ct is threshold cycle) method upon normalization to RNA polymerase II (Pol2) expression. Radh10, retinol dehydrogenase 10; Lrrc32, leucine-rich repeat containing 32; Entpd2, ectonucleoside triphosphate diphosphohydrolase 2; Cp, ceruloplasmin; Arsi, arylsulfatase; Alpl, alkaline phosphatase; Ptc1, patched 1. C: induction of selected Shh-responsive genes at the protein level. Representative Western blots of whole cell lysates of NRVMs stimulated during 48 h in medium containing 0.5% FBS and vehicle, 2.5 mg/l Shh, or Shh plus 0.5 μmol/L KAAD-CP are shown. The experiment was independently repeated 3 times.
Canonical Hh signaling in cardiomyocytes requires Gi proteins.
Since canonical Hh signaling requires Smo coupling to Gi in some but not all cell types, we sought to determine its function in cardiomyocytes. To this end, NRVMs were infected with adenoviral particles encoding LacZ (control) or GiCT, which blocks the GPCR-Gi protein interaction and thus acts as a dominant negative inhibitor of Gi signaling (8). Using a multiplicity of infection of 100, transduction efficiency was near 100%, as determined by X-gal staining in AdV-LacZ-infected NRVMs (data no shown). Western blot analysis against the HA tag of GiCT revealed robust expression of the transgene 24 h postinfection (Fig. 3A). At this time, cells were stimulated with 2.5 mg/l recombinant Shh for additional 24 h, and mRNA levels of Gli1 and Pol2 were determined by quantitative PCR. Expression of GiCT impaired the response to Shh with >50% inhibition of the maximal Gli1 induction compared with β-galactosidase-expressing cells (Fig. 3B). Hence, our results convincingly suggest that impaired Gi protein activation in primary NRVMs results in defective canonical Hh signal transduction.
Fig. 3.
Canonical Hh signaling in cardiomyocytes requires Gi protein activation. A: expression of the hemagglutinin (HA)-tagged GiCT peptide in NRVMs transduced with AdV-GiCT or control AdV-LacZ (multiplicity of infection: 100). Representative Western blots of HA and β-actin (loading control) are shown. B: NRVMs were cultured in the presence of the indicated adenoviral particles at a multiplicity of infection of 100 for 24 h in complete medium and then switched to low serum medium supplemented with 2.5 mg/l Shh or vehicle. After 24 h, total RNA was isolated and processed for quantitative RT-PCR of Gli1 and the housekeeping gene Pol2. Results are means ± SE of the normalized ratio of Gli1 to S15 from 3 independent experiments; n = 3. *P < 0.05. C: quantitation of intracellular cAMP levels in NRVMs transduced with control AdV-LacZ or AdV-GiCT stimulated for 5 min with vehicle or 10 μmol/L isoproterenol (Iso) in combination with 2.5 mg/l Shh or 5 μmol/l PUR with or without the smoothened inhibitor KAAD-CP (0.5 μmol/l). Results are means ± SE of 3 independent experiments calculated against a standard curve; n = 3. *Vehicle vs. Iso, P < 0.001; *Iso vs. Iso + Shh or Iso + PUR, P < 0.05. NS, not significant.
Activation of Smo causes an acute reduction in intracellular cAMP levels.
To further demonstrate that Smo activates Gi proteins in cardiomyocytes, we quantified the activity of adenylate cyclase, a well-characterized target of Gi. Gi proteins inhibit adenylate cyclase, leading to a reduction in cAMP generation. Since basal levels of cAMP are low, inhibition of adenylate cyclase is better observed by competition with an activator. Thus, we first incubated cells with vehicle, Shh, or purmorphamine and then stimulated cAMP production with the β-adrenergic agonist Iso for 5 min. Total cAMP levels were then quantified in cell lysates in the presence of phosphodiesterase inhibitors by enzyme immunoassay. As shown in Fig. 3C, left, Iso-stimulated cAMP production was significantly reduced by simultaneous activation of Smo with Shh or purmorphamine, indicating that Smo opposes adenylate cyclase activity in cardiomyocytes. This effect was specific since cotreatment with the Smo inhibitor KAAD-cyclopamine significantly restored cAMP production when cells were stimulated with either Shh or purmorphamine, although in the latter case to a lesser degree, as expected (Fig. 3C). Moreover, introduction of GiCT by adenoviral delivery prevented the reduction in adenylate cyclase activity by Shh or purmorphamine (Fig. 3C, right). Together, these observations demonstrate that Smo activates Gi proteins and that this translates into acute inhibition of adenylate cyclase and reduction of cAMP. Since PKA is a negative regulator of the mammalian Hh pathway, it is likely that Gi proteins are required for Gli transcriptional activation in cardiomyocytes to reduce the activity of PKA activity by lowering the intracellular cAMP concentration.
Canonical Hh signaling is reduced in the myocardium of transgenic GiCT mice.
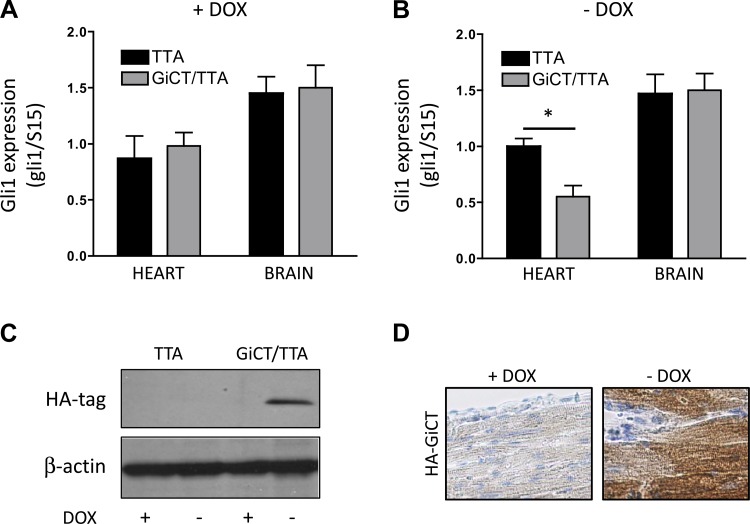
Mice engineered to express the GiCT peptide under the control of the α-MHC promoter in a Tet-off inducible manner (GiCT/TTA mice) serve as a model to study coupling of GPCRs to Gi proteins in the myocardium. Since Smo, like most GPCRs, couples to more than one Gi family member, the use of a pan-Gi protein family inhibitor provides a better model to study Smo-Gi coupling than individual knockouts. Expression of GiCT in the heart does not affect cardiac function under nonstress conditions, as previously reported by DeGeorge et al. (22). To ascertain if postnatal canonical Hh signaling in the heart depends on Gi activation, we compared control mice (TTA mice) with GiCT/TTA mice in which GiCT expression in the α-MHC domain is regulated by the administration of doxycycline. After receiving doxycycline in food for 2 wk, TTA and GiCT/TTA mice expressed comparable levels of the Hh target gene Gli1 in the heart and brain (Fig. 4A). However, when GiCT expression was induced by removal of doxycycline from the diet, Gli1 levels were specifically reduced in the heart (∼50% of control animals, n = 6) but not in the brain of GiCT/TTA animals, demonstrating a local effect of GiCT on Hh signaling in the myocardium (Fig. 4B). Selective expression of the transgene in the absence of doxycycline in GiCT/TTA hearts was corroborated by the presence of an ∼10-kDa HA-reactive band by Western blot analysis (Fig. 4C), and restricted localization to cardiomyocytes was verified by immunostaining of the left ventricle (Fig. 4D). In addition, we evaluated the reversibility of the effect of Gi inhibition on canonical Hh signaling by resupplementing GiCT/TTA and control mice with doxycycline for 30 days. The results indicate that Gli1 expression in GiCT/TTA mice was rescued by repression of the transgene (data not shown). Altogether, these results suggest that Gi proteins mediate endogenous Hh signaling in the myocardium.
Fig. 4.
Canonical Hh signaling in the intact heart requires Gi protein activation. A: Gli1 expression in whole heart or brain tissue of control (TTA) or GiCT/TTA mice when GiCT is repressed by the administration of doxycycline (Dox). B: Gli1 expression in whole heart or brain tissue of control (TTA) or GiCT/TTA mice when GiCT is induced by the removal of Dox. n = 6. *P < 0.005. C: representative Western blots showing expression of HA-tagged GiCT peptide in whole hearts from TTA and GiCT/TTA mice supplemented or not with Dox−. D: HA immunostaining in the myocardium of GiCT/TTA mice supplemented or not with Dox−. Sections were counterstained with hematoxylin.
Activation of the Hh pathway by ischemic injury is impaired in the absence of Gi signaling.
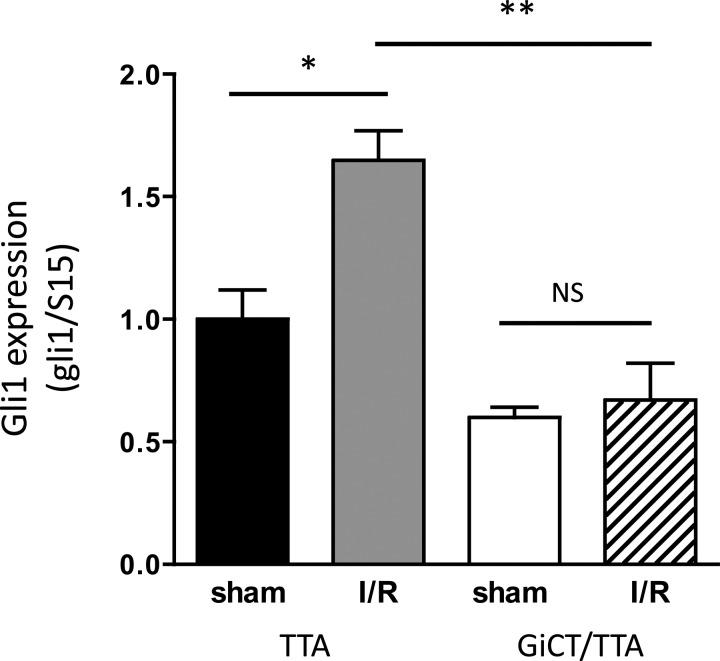
Hypoxia and ischemic injury have been previously reported to induce the Hh pathway in the heart (3, 14). To evaluate if impaired Gi signaling perturbs the upregulation of the Hh pathway in response to ischemia, we subjected control TTA and GiCT/TTA mice to 30-min ischemic injury by ligation of the LAD artery followed by 72 h of reperfusion or sham surgery. The affected ventricular area was dissected, and mRNA was extracted for analysis of Gli1 expression by quantitative PCR. Increased Gli1 expression (∼65%, n = 6) was observed in hearts of TTA mice subjected to the I/R protocol but not in GiCT/TTA (Fig. 5), which also had lower basal Gli1 expression (as shown in Fig. 4B). These observations indicate a failure to activate the Hh pathway under ischemic conditions in the myocardium in vivo by defective Gi signaling.
Fig. 5.
Induction of Hh target genes during ischemia-reperfusion (I/R) is dependent on Gi proteins. Expression of Gli1 was normalized to S15 in the left ventricle of TTA and GiCT/TTA mice without Doxy supplementation that were subjected to sham surgery or to 30-min ischemia by ligation of the left anterior descending coronary artery followed by 72 h of reperfusion (I/R). n = 6. *P < 0.01; **P < 0.001.
DISCUSSION
The role of Smo as a GPCR for canonical Hh signaling is still under debate, as many cell types do not require the activation of Gi to transduce the activation of Smo to Gli transcription factors. Even very closely related cell types, like mouse embryonic fibroblasts and NIH-3T3 fibroblasts, show a different need of Gi (17, 22, 26). In the present study, we show that Hh signaling in cardiac muscle cells is dependent on activation of Gi by Smo, both in vitro and in vivo. A substantial impairment of Hh signaling is evident in whole hearts of mice expressing the GiCT peptide. However, there is ∼50% remaining activity that is likely attributable to cardiac fibroblasts and perivascular cells, which do not express the transgene and have been shown to respond readily to Shh (15, 21). This finding underscores the important contribution of cardiomyocytes to the additive response of different cell types in the heart to Shh. Moreover, our results indicate that Gi plays a central role in the physiological upregulation of the Gli transcriptional program in the heart in response to transient ischemia and that such upregulation occurs mainly in cardiac muscle cells. It is noteworthy that GiCT/TTA mice subjected to I/R injury were reported to have a significant reduction in cardiac contractile performance and exhibited increased infarct size and cardiomyocyte apoptosis compared with nontransgenic mice, suggesting that failure to activate Hh signaling impairs a cardioprotective mechanism (8).
Our findings reveal that Hh signaling modulates a select number of genes in cardiomyocytes. We observed upregulation of classical Gli responsive genes Gli1, Ptc1, and Alpl but also of other genes never reported to be under the control of Smo. In this regard, it was very important to analyze only those genes stimulated by Shh that are sensitive to Smo inhibition with KAAD-cyclopamine. The highest induced gene was the acute phase response gene Cp. Cp is a serum protein that belongs to the family of α2-globulins, and it is increased in patients with after acute myocardial infarction and uncompensated heart failure (5, 13). Cp acts as an antioxidant and could be part of the cardioprotective mechanism against free radical-mediated injury after reperfusion. While Cp is not a common Hh-responsive gene, a single microarray study (12) reported the induction of Cp by Shh in MNS-70 neuroepithelial cells. Another myocardium-specific upregulated gene is Endtp2. Entdp2 is the isoform with the highest expression in the rat left ventricle (28). Entdp enzymes convert extracellular ATP and ADP to AMP and, thus, reduce P2 receptor activation. The AMP produced is then converted into adenosine by ecto-5′-nucleotidase. In turn, adenosine activates P1 receptors, initiating cellular responses often opposite to those elicited by ATP. It is highly suggestive that Shh induces another Entdp family member, Alpl, in cardiomyocytes. We speculate that Shh signaling might cause a reduction of extracellular ATP/ADP and a selective shift from P2 receptors toward P1 receptor signaling via upregulation of Alpl and Entdp2, resulting in vasodilation and bradycardia.
Our findings are supported anecdotal evidence of Gi protein signaling and Hh signaling dysfunction in a mouse model of type I diabetes. Streptozotocin-treated mice exhibit decreased levels of Gαi proteins in the myocardium, similar to the human diabetic heart (19, 32). In these animals, the normal upregulation of the Hh pathway after myocardial infarction is impaired (33). The phenotype was associated with increased cardiac dysfunction and reduced capillary density and can be partly restored by administration of the Smo agonist SAG1.3. Therefore, a possible explanation is that a reduction in Gi protein signaling could inhibit the Hh pathway in the heart of diabetic mice.
In summary, our study highlights the important role of Gi proteins as mediators of physiological basal canonical Hh signaling in the myocardium and in the pathological upregulation of the pathway during I/R injury. While the role of Smo as a GPCR during embryonic development remains questionable, our findings support a fundamental role of G proteins in Hh signaling in adult tissues. Future studies will shed light on the role of Smo as a GPCR in other postnatal organs.
GRANTS
This work was sponsored by National Institute of General Medical Sciences Grant R01-GM-088256 (to N. A. Riobo).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
Author contributions: C.J.C., L.C., S.A., J.I.G., E.G., and N.A.R. performed experiments; C.J.C., L.C., S.A., and N.A.R. analyzed data; C.J.C., W.J.K., and N.A.R. interpreted results of experiments; C.J.C., L.C., S.A., J.I.G., E.G., W.J.K., and N.A.R. approved final version of manuscript; L.C. and N.A.R. conception and design of research; S.A. and N.A.R. prepared figures; N.A.R. drafted manuscript; N.A.R. edited and revised manuscript.
REFERENCES
- 1.Ahmed RP, Haider KH, Shujia J, Afzal MR, Ashraf M. Sonic hedgehog gene delivery to the rodent heart promotes angiogenesis via iNOS/netrin-1/PKC pathway. PLOS ONE 5: e8576, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Belgacem YH, Borodinsky LN. Sonic hedgehog signaling is decoded by calcium spike activity in the developing spinal cord. Proc Natl Acad Sci USA 108: 4482–4487, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bijlsma MF, Leenders PJ, Janssen BJ, Peppelenbosch MP, Ten Cate H, Spek CA. Endogenous hedgehog expression contributes to myocardial ischemia-reperfusion-induced injury. Exp Biol Med (Maywood) 233: 989–996, 2008 [DOI] [PubMed] [Google Scholar]
- 4.Brennan D, Chen X, Cheng L, Mahoney M, Riobo NA. Noncanonical hedgehog signaling. Vitam Horm 88: 55–72, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Brunetti ND, Pellegrino PL, Correale M, De Gennaro L, Cuculo A, Di Biase M. Acute phase proteins and systolic dysfunction in subjects with acute myocardial infarction. J Thromb Thrombolysis 26: 196–202, 2008 [DOI] [PubMed] [Google Scholar]
- 6.Chinchilla P, Xiao L, Kazanietz MG, Riobo NA. Hedgehog proteins activate pro-angiogenic responses in endothelial cells through non-canonical signaling pathways. Cell Cycle 9: 570–579, 2010 [DOI] [PubMed] [Google Scholar]
- 7.Choi WY, Gemberling M, Wang J, Holdway JE, Shen MC, Karlstrom RO, Poss KD. In vivo monitoring of cardiomyocyte proliferation to identify chemical modifiers of heart regeneration. Development 140: 660–666, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.DeGeorge BR, Jr, Gao E, Boucher M, Vinge LE, Martini JS, Raake PW, Chuprun JK, Harris DM, Kim GW, Soltys S, Eckhart AD, Koch WJ. Targeted inhibition of cardiomyocyte Gi signaling enhances susceptibility to apoptotic cell death in response to ischemic stress. Circulation 117: 1378–1387, 2008 [DOI] [PubMed] [Google Scholar]
- 9.Dyer LA, Kirby ML. Sonic hedgehog maintains proliferation in secondary heart field progenitors and is required for normal arterial pole formation. Dev Biol 330: 305–317, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dyer LA, Makadia FA, Scott A, Pegram K, Hutson MR, Kirby ML. BMP signaling modulates hedgehog-induced secondary heart field proliferation. Dev Biol 348: 167–176, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gao E, Koch WJ. A novel and efficient model of coronary artery ligation in the mouse. Methods Mol Biol 1037: 299–311, 2013 [DOI] [PubMed] [Google Scholar]
- 12.Kato M, Seki N, Sugano S, Hashimoto K, Masuho Y, Muramatsu M, Kaibuchi K, Nakafuku M. Identification of sonic hedgehog-responsive genes using cDNA microarray. Biochem Biophys Res Commun 289: 472–478, 2001 [DOI] [PubMed] [Google Scholar]
- 13.Kaya Z, Kaya BC, Sezen H, Bilinc H, Asoglu R, Yildiz A, Taskin A, Yalcin S, Sezen Y, Aksoy N. Serum ceruloplasmin levels in acute decompensated heart failure. Clin Ter 164: e187–91, 2013 [DOI] [PubMed] [Google Scholar]
- 14.Kusano KF, Pola R, Murayama T, Curry C, Kawamoto A, Iwakura A, Shintani S, Ii M, Asai J, Tkebuchava T, Thorne T, Takenaka H, Aikawa R, Goukassian D, von Samson P, Hamada H, Yoon YS, Silver M, Eaton E, Ma H, Heyd L, Kearney M, Munger W, Porter JA, Kishore R, Losordo DW. Sonic hedgehog myocardial gene therapy: tissue repair through transient reconstitution of embryonic signaling. Nat Med 11: 1197–1204, 2005 [DOI] [PubMed] [Google Scholar]
- 15.Lavine KJ, Kovacs A, Ornitz DM. Hedgehog signaling is critical for maintenance of the adult coronary vasculature in mice. J Clin Invest 118: 2404–2414, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lavine KJ, White AC, Park C, Smith CS, Choi K, Long F, Hui CC, Ornitz DM. Fibroblast growth factor signals regulate a wave of hedgehog activation that is essential for coronary vascular development. Genes Dev 20: 1651–1666, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Low WC, Wang C, Pan Y, Huang XY, Chen JK, Wang B. The decoupling of smoothened from Gαi proteins has little effect on Gli3 protein processing and hedgehog-regulated chick neural tube patterning. Dev Biol 321: 188–196, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Martinez-Chinchilla P, Riobo NA. Purification and bioassay of hedgehog ligands for the study of cell death and survival. Methods Enzymol 446: 189–204, 2008 [DOI] [PubMed] [Google Scholar]
- 19.Matsuda N, Hattori Y, Gando S, Watanuki S, Kemmotsu O, Kanno M. Differential gene transcriptional regulation of Gi isoforms and Gs protein expression in diabetic rat hearts. Naunyn Schmiedebergs Arch Pharmacol 361: 53–60, 2000 [DOI] [PubMed] [Google Scholar]
- 20.Pola R, Ling LE, Aprahamian TR, Barban E, Bosch-Marce M, Curry C, Corbley M, Kearney M, Isner JM, Losordo DW. Postnatal recapitulation of embryonic hedgehog pathway in response to skeletal muscle ischemia. Circulation 108: 479–485, 2003 [DOI] [PubMed] [Google Scholar]
- 21.Pola R, Ling LE, Silver M, Corbley MJ, Kearney M, Blake Pepinsky R, Shapiro R, Taylor FR, Baker DP, Asahara T, Isner JM. The morphogen sonic hedgehog is an indirect angiogenic agent upregulating two families of angiogenic growth factors. Nat Med 7: 706–711, 2001 [DOI] [PubMed] [Google Scholar]
- 22.Polizio AH, Chinchilla P, Chen X, Kim S, Manning DR, Riobo NA. Heterotrimeric Gi proteins link hedgehog signaling to activation of Rho small GTPases to promote fibroblast migration. J Biol Chem 286: 19589–19596, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Polizio AH, Chinchilla P, Chen X, Manning DR, Riobo NA. Sonic hedgehog activates the GTPases Rac1 and RhoA in a Gli-independent manner through coupling of smoothened to Gi proteins. Sci Signal 4: pt7, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Riobo NA, Lu K, Ai X, Haines GM, Emerson CP., Jr Phosphoinositide 3-kinase and Akt are essential for sonic hedgehog signaling. Proc Natl Acad Sci USA 103: 4505–4510, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Riobo NA, Lu K, Emerson CP., Jr Hedgehog signal transduction: signal integration and cross talk in development and cancer. Cell Cycle 5: 1612–1615, 2006 [DOI] [PubMed] [Google Scholar]
- 26.Riobo NA, Saucy B, Dilizio C, Manning DR. Activation of heterotrimeric G proteins by smoothened. Proc Natl Acad Sci USA 103: 12607–12612, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Robbins DJ, Fei DL, Riobo NA. The hedgehog signal transduction network. Sci Signal 5: re6, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Rücker B, Almeida ME, Libermann TA, Zerbini LF, Wink MR, Sarkis JJ. E-NTPDases and ecto-5′-nucleotidase expression profile in rat heart left ventricle and the extracellular nucleotide hydrolysis by their nerve terminal endings. Life Sci 82: 477–486, 2008 [DOI] [PubMed] [Google Scholar]
- 29.Sinha S, Chen JK. Purmorphamine activates the hedgehog pathway by targeting smoothened. Nat Chem Biol 2: 29–30, 2006 [DOI] [PubMed] [Google Scholar]
- 30.Ueda K, Takano H, Niitsuma Y, Hasegawa H, Uchiyama R, Oka T, Miyazaki M, Nakaya H, Komuro I. Sonic hedgehog is a critical mediator of erythropoietin-induced cardiac protection in mice. J Clin Invest 120: 2016–2029, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wang B, Fallon JF, Beachy PA. Hedgehog-regulated processing of Gli3 produces an anterior/posterior repressor gradient in the developing vertebrate limb. Cell 100: 423–434, 2000 [DOI] [PubMed] [Google Scholar]
- 32.Wichelhaus A, Russ M, Petersen S, Eckel J. G protein expression and adenylate cyclase regulation in ventricular cardiomyocytes from STZ-diabetic rats. Am J Physiol Heart Circ Physiol 267: H548–H555, 1994 [DOI] [PubMed] [Google Scholar]
- 33.Xiao Q, Hou N, Wang YP, He LS, He YH, Zhang GP, Yi Q, Liu SM, Chen MS, Luo JD. Impaired sonic hedgehog pathway contributes to cardiac dysfunction in type 1 diabetic mice with myocardial infarction. Cardiovasc Res 95: 507–516, 2012 [DOI] [PubMed] [Google Scholar]