Figure 31.
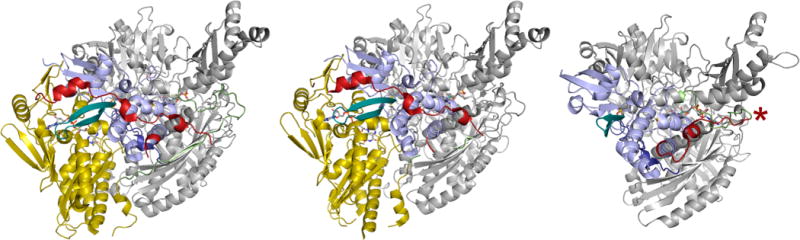
A comparison of the polypeptide trace in bovine xanthine dehydrogenase (PDB F1O4), mouse aldehyde oxidase (PDB 3ZYV), and D. gigas aldehyde oxidoreductase (PDB 1VLB). The iron–sulfur domains (of one subunit each of the homodimers) are in blue, the FAD domains (when present) are in yellow, and the molybdenum domains are in gray. The linker between the Fe/S and FAD domains in the first two structures is in red, and the linker between the FAD and Mo domains is in green. In the bacterial enzyme at right, the single linker between the Fe/S and Mo domains is in red and green, with the approximate point of insertion of the FAD domain indicated by the red asterisk (far right). The β-turn of the first Fe/S domain that is elongated in the eukaryotic enzymes is shown in teal.
