Abstract
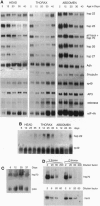
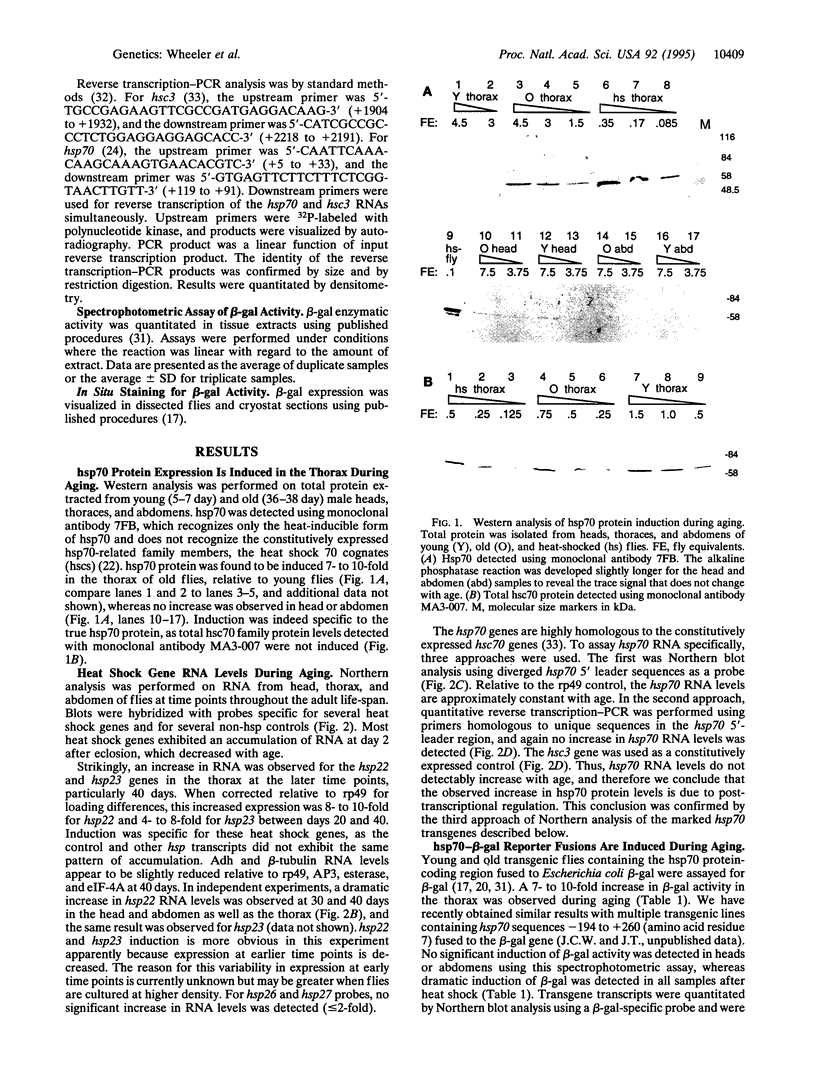
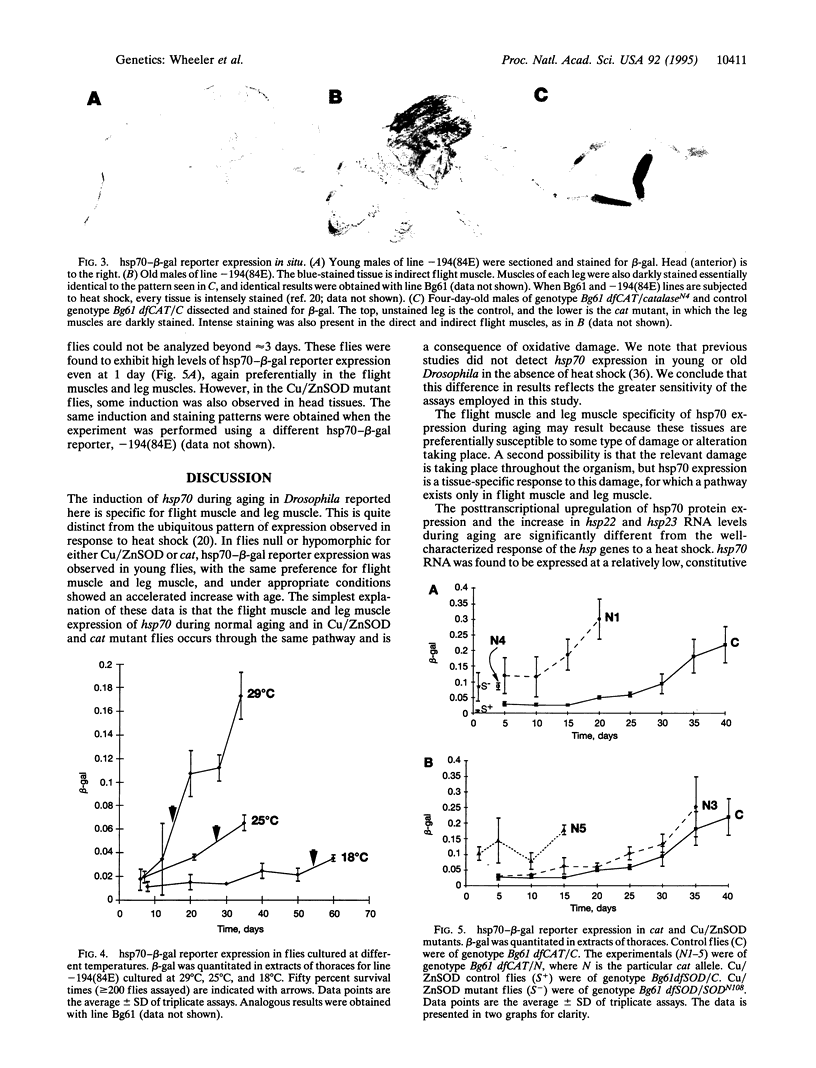
Induction of Drosophila hsp70 protein was detected during aging in flight muscle and leg muscle in the absence of heat shock, using an hsp70-specific monoclonal antibody, and in transgenic flies containing hsp70-beta-galactosidase fusion protein reporter constructs. While hsp70 and reporter proteins were induced during aging, hsp70 message levels were not, indicating that aging-specific induction is primarily posttranscriptional. In contrast, hsp22 and hsp23 were found to be induced during aging at the RNA level and with a broader tissue distribution. The same muscle-specific hsp70 reporter expression pattern was observed in young flies mutant for catalase (H2O2:H2O2 oxidoreductase, EC 1.11.1.6). In catalase (cat) hypomorphic lines where flies survived to older ages, the time course of hsp70 reporter expression during aging was accelerated, and the initial and ultimate levels of expression were increased. The hsp70 reporter was also induced in young flies mutant for copper/zinc superoxide dismutase (superoxide:superoxide oxidoreductase, EC 1.15.1.1). Taken together, the results suggest that aging-specific hsp70 expression may be a result of oxidative damage.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ananthan J., Goldberg A. L., Voellmy R. Abnormal proteins serve as eukaryotic stress signals and trigger the activation of heat shock genes. Science. 1986 Apr 25;232(4749):522–524. doi: 10.1126/science.3083508. [DOI] [PubMed] [Google Scholar]
- Benyajati C., Spoerel N., Haymerle H., Ashburner M. The messenger RNA for alcohol dehydrogenase in Drosophila melanogaster differs in its 5' end in different developmental stages. Cell. 1983 May;33(1):125–133. doi: 10.1016/0092-8674(83)90341-0. [DOI] [PubMed] [Google Scholar]
- Bialojan S., Falkenburg D., Renkawitz-Pohl R. Characterization and developmental expression of beta tubulin genes in Drosophila melanogaster. EMBO J. 1984 Nov;3(11):2543–2548. doi: 10.1002/j.1460-2075.1984.tb02170.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Craig E. A., Gross C. A. Is hsp70 the cellular thermometer? Trends Biochem Sci. 1991 Apr;16(4):135–140. doi: 10.1016/0968-0004(91)90055-z. [DOI] [PubMed] [Google Scholar]
- DiDomenico B. J., Bugaisky G. E., Lindquist S. The heat shock response is self-regulated at both the transcriptional and posttranscriptional levels. Cell. 1982 Dec;31(3 Pt 2):593–603. doi: 10.1016/0092-8674(82)90315-4. [DOI] [PubMed] [Google Scholar]
- Dorn R., Morawietz H., Reuter G., Saumweber H. Identification of an essential Drosophila gene that is homologous to the translation initiation factor eIF-4A of yeast and mouse. Mol Gen Genet. 1993 Feb;237(1-2):233–240. doi: 10.1007/BF00282805. [DOI] [PubMed] [Google Scholar]
- Gershon H., Gershon D. Detection of inactive enzyme molecules in ageing organisms. Nature. 1970 Sep 19;227(5264):1214–1217. doi: 10.1038/2271214a0. [DOI] [PubMed] [Google Scholar]
- Goff S. A., Goldberg A. L. Production of abnormal proteins in E. coli stimulates transcription of lon and other heat shock genes. Cell. 1985 Jun;41(2):587–595. doi: 10.1016/s0092-8674(85)80031-3. [DOI] [PubMed] [Google Scholar]
- Griswold C. M., Matthews A. L., Bewley K. E., Mahaffey J. W. Molecular characterization and rescue of acatalasemic mutants of Drosophila melanogaster. Genetics. 1993 Jul;134(3):781–788. doi: 10.1093/genetics/134.3.781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ingolia T. D., Craig E. A., McCarthy B. J. Sequence of three copies of the gene for the major Drosophila heat shock induced protein and their flanking regions. Cell. 1980 Oct;21(3):669–679. doi: 10.1016/0092-8674(80)90430-4. [DOI] [PubMed] [Google Scholar]
- Karlik C. C., Coutu M. D., Fyrberg E. A. A nonsense mutation within the act88F actin gene disrupts myofibril formation in Drosophila indirect flight muscles. Cell. 1984 Oct;38(3):711–719. doi: 10.1016/0092-8674(84)90266-6. [DOI] [PubMed] [Google Scholar]
- Kelley M. R., Venugopal S., Harless J., Deutsch W. A. Antibody to a human DNA repair protein allows for cloning of a Drosophila cDNA that encodes an apurinic endonuclease. Mol Cell Biol. 1989 Mar;9(3):965–973. doi: 10.1128/mcb.9.3.965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lindquist S., Craig E. A. The heat-shock proteins. Annu Rev Genet. 1988;22:631–677. doi: 10.1146/annurev.ge.22.120188.003215. [DOI] [PubMed] [Google Scholar]
- Lindquist S., Petersen R. Selective translation and degradation of heat-shock messenger RNAs in Drosophila. Enzyme. 1990;44(1-4):147–166. doi: 10.1159/000468754. [DOI] [PubMed] [Google Scholar]
- Lis J. T., Simon J. A., Sutton C. A. New heat shock puffs and beta-galactosidase activity resulting from transformation of Drosophila with an hsp70-lacZ hybrid gene. Cell. 1983 Dec;35(2 Pt 1):403–410. doi: 10.1016/0092-8674(83)90173-3. [DOI] [PubMed] [Google Scholar]
- Mackay W. J., Bewley G. C. The genetics of catalase in Drosophila melanogaster: isolation and characterization of acatalasemic mutants. Genetics. 1989 Jul;122(3):643–652. doi: 10.1093/genetics/122.3.643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miquel J., Lundgren P. R., Bensch K. G., Atlan H. Effects of temperature on the life span, vitality and fine structure of Drosophila melanogaster. Mech Ageing Dev. 1976 Sep-Oct;5(5):347–370. doi: 10.1016/0047-6374(76)90034-8. [DOI] [PubMed] [Google Scholar]
- Niedzwiecki A., Kongpachith A. M., Fleming J. E. Aging affects expression of 70-kDa heat shock proteins in Drosophila. J Biol Chem. 1991 May 15;266(14):9332–9338. [PubMed] [Google Scholar]
- O'Connell P. O., Rosbash M. Sequence, structure, and codon preference of the Drosophila ribosomal protein 49 gene. Nucleic Acids Res. 1984 Jul 11;12(13):5495–5513. doi: 10.1093/nar/12.13.5495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oakeshott J. G., Collet C., Phillis R. W., Nielsen K. M., Russell R. J., Chambers G. K., Ross V., Richmond R. C. Molecular cloning and characterization of esterase-6, a serine hydrolase of Drosophila. Proc Natl Acad Sci U S A. 1987 May;84(10):3359–3363. doi: 10.1073/pnas.84.10.3359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orr W. C., Sohal R. S. Extension of life-span by overexpression of superoxide dismutase and catalase in Drosophila melanogaster. Science. 1994 Feb 25;263(5150):1128–1130. doi: 10.1126/science.8108730. [DOI] [PubMed] [Google Scholar]
- Phillips J. P., Campbell S. D., Michaud D., Charbonneau M., Hilliker A. J. Null mutation of copper/zinc superoxide dismutase in Drosophila confers hypersensitivity to paraquat and reduced longevity. Proc Natl Acad Sci U S A. 1989 Apr;86(8):2761–2765. doi: 10.1073/pnas.86.8.2761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reveillaud I., Niedzwiecki A., Bensch K. G., Fleming J. E. Expression of bovine superoxide dismutase in Drosophila melanogaster augments resistance of oxidative stress. Mol Cell Biol. 1991 Feb;11(2):632–640. doi: 10.1128/mcb.11.2.632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubin D. M., Mehta A. D., Zhu J., Shoham S., Chen X., Wells Q. R., Palter K. B. Genomic structure and sequence analysis of Drosophila melanogaster HSC70 genes. Gene. 1993 Jun 30;128(2):155–163. doi: 10.1016/0378-1119(93)90558-k. [DOI] [PubMed] [Google Scholar]
- Simon J. A., Lis J. T. A germline transformation analysis reveals flexibility in the organization of heat shock consensus elements. Nucleic Acids Res. 1987 Apr 10;15(7):2971–2988. doi: 10.1093/nar/15.7.2971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simon J. A., Sutton C. A., Lobell R. B., Glaser R. L., Lis J. T. Determinants of heat shock-induced chromosome puffing. Cell. 1985 Apr;40(4):805–817. doi: 10.1016/0092-8674(85)90340-x. [DOI] [PubMed] [Google Scholar]
- Sohal R. S., Agarwal S., Dubey A., Orr W. C. Protein oxidative damage is associated with life expectancy of houseflies. Proc Natl Acad Sci U S A. 1993 Aug 1;90(15):7255–7259. doi: 10.1073/pnas.90.15.7255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stadtman E. R. Protein oxidation and aging. Science. 1992 Aug 28;257(5074):1220–1224. doi: 10.1126/science.1355616. [DOI] [PubMed] [Google Scholar]
- Takahashi A., Philpott D. E., Miquel J. Electron microscope studies on aging Drosophila melanogaster. I. Dense bodies. J Gerontol. 1970 Jul;25(3):210–217. doi: 10.1093/geronj/25.3.210. [DOI] [PubMed] [Google Scholar]
- Velazquez J. M., Lindquist S. hsp70: nuclear concentration during environmental stress and cytoplasmic storage during recovery. Cell. 1984 Mar;36(3):655–662. doi: 10.1016/0092-8674(84)90345-3. [DOI] [PubMed] [Google Scholar]