Summary
Adult stem cells reside in specialized regulatory microenvironments, or niches, where local signals ensure stem cell maintenance. The Drosophila testis contains a well-characterized niche wherein signals from post-mitotic hub cells promote maintenance of adjacent germline stem cells and somatic cyst stem cells (CySCs). Hub cells were considered to be terminally differentiated; here we show that they can give rise to CySCs. Genetic ablation of CySCs triggers hub cells to transiently exit quiescence, delaminate from the hub, and convert into functional CySCs. Ectopic Cyclin D-Cdk4 expression in hub cells is also sufficient to trigger their conversion into CySCs. In both cases, this conversion causes the formation of multiple ectopic niches over time. Therefore, our work provides a model for understanding how oncogenic mutations in quiescent niche cells could promote loss of quiescence, changes in cell fate, and aberrant niche expansion more generally.
Keywords: stem cell, niche, spermatogenesis, loss of quiescence, transdifferentiation, Cyclin D-Cdk4
Introduction
Niches are specialized microenvironments that regulate tissue-specific stem cells via local signals. Understanding how niches ensure stem cell renewal, particularly in response to age or tissue damage, remains challenging due to the complexity of most niches (Hsu and Fuchs, 2012). The cells within a tumor that sustain long-term propagation of cancer (cancer stem cells) are also thought to reside in niches, but the biogenesis of cancer stem cells and their niches is poorly understood (Visvader and Lindeman, 2012).
One of the best characterized niches is found in the Drosophila testis apex, where a cluster of quiescent somatic cells called the hub creates a niche that maintains adjacent germline stem cells (GSCs) and somatic cyst stem cells (CySCs) (Fig. 1A) (de Cuevas and Matunis, 2011). GSCs and CySCs divide asymmetrically, producing new stem cells (self-renewal) and daughters that are displaced from the hub and differentiate. GSCs give rise to gonialblasts, which undergo four rounds of mitosis with incomplete cytokinesis to form clusters of spermatogonia, while CySCs give rise to postmitotic cyst cells, which envelop dividing germ cells and sustain their development. GSCs lost through damage or aging are typically replaced by remaining GSCs but can also arise from dedifferentiation of spermatogonia (Brawley and Matunis, 2004; Cheng et al., 2008; Sheng et al., 2009). How lost CySCs are replaced, however, is not understood.
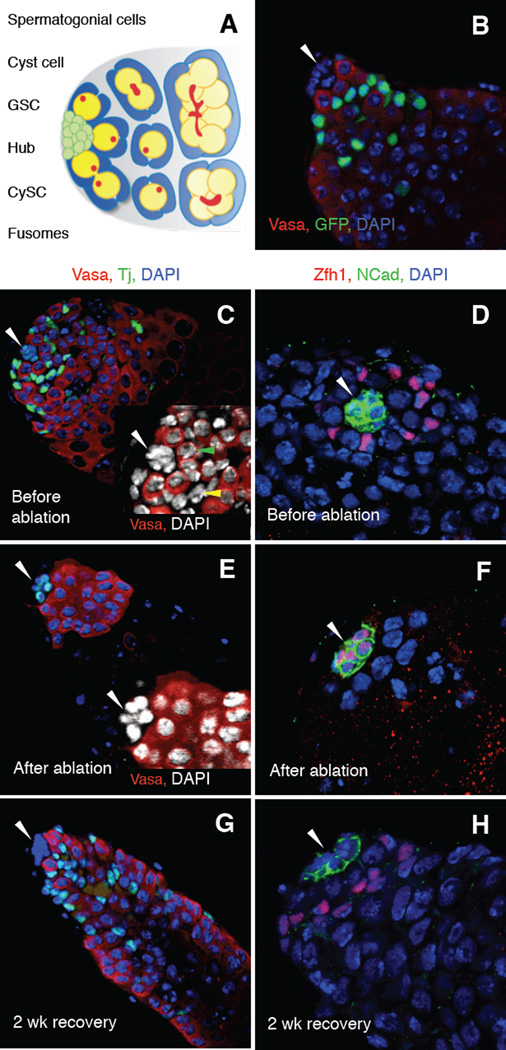
Figure 1. Recovery of functional CySCs after complete genetic ablation of CySCs and early cyst cells in the adult testis.
(A) The Drosophila testis apex. About 10 germline stem cells (GSCs, yellow) adhere to the hub (green), a cluster of 15–20 quiescent somatic cells. GSCs divide asymmetrically, producing new stem cells (self-renewal) and daughters that are displaced from the hub and differentiate. Differentiating daughters form clusters of spermatogonia, which differentiate into spermatocytes. Somatic cyst stem cells (CySCs, blue) flank each GSC and adhere to the hub via thin cytoplasmic extensions. CySCs self-renew and produce cyst cells, which envelop differentiating germ cells. The fusome (red) is round in GSCs and gonialblasts, and elongated or branched in spermatogonial clusters. (B–H) Single confocal sections through the testis apex immunostained with antibodies as indicated. Nuclei are counterstained with 4',6-diamidino-2-phenylindole (DAPI, blue). The hub is indicated (arrowhead). (B) Testis expressing Green fluorescent protein (GFP) under the control of the c587-Gal4 driver immunostained with anti-GFP (green; CySCs and early cyst cells associated with spermatogonia) and anti-Vasa (red; germ cells). (C–H) Testes from c587-Gal4; UAS-grim/+; tub-Gal80ts/+ flies (referred to as c587-Gal4>grim hereafter) immunostained with (C, E, G) anti-Vasa (red; germ cells) and anti-Tj (green; hub cells, CySCs and early cyst cells) or (D, F, H) anti-Zfh1 (red; hub cells, CySCs and their immediate daughters) and anti-CadN (green; hub cells). (C–D) Before Grim expression (18°C), testes appear wild type: Tj-positive and Zfh1-positive cells are found within all testes (n = 249 and 64 testes, respectively). (C, inset) A single CySC (yellow arrow) and GSC (green arrow) are indicated. (E–F) After 2 days of Grim expression (31°C), all CySCs and early cyst cells are completely ablated. No Tj-positive or Zfh1-positive cells are found outside the hub (n = 728 and 85 testes, respectively). All remaining cells outside the hub are DAPI-positive/Vasa-positive; no Vasa-negative cells are observed (E, inset). Incidentally, at 31°C, hub cells express higher levels of Tj and Zfh1; this is due to temperature, not CySC ablation, as it also occurs in control testes at 31°C (not shown). (G–H) After ablation (2 days at 31°C) and 2 weeks of recovery (18 °C), testes can regain CySCs and early cyst cells. Scale bars, 20 µm. See also Figure S1 and Table S1.
Here, we show that quiescent hub cells can convert into functional CySCs after complete genetic ablation of CySCs or forced expression of Cyclin D and Cyclin-dependent kinase 4 (Cdk4) in the hub, but not in response to partial loss of CySCs. As a consequence of this conversion, multiple ectopic niches can form in the testis over time. Our results suggest that hub cells can give rise to CySCs and provide a simple model for understanding how lost stem cell populations can be replaced by activation of quiescent niche cells.
Results
CySCs in the adult testis can be genetically ablated
To ask if CySCs can be restored after ablation, we first established conditions to genetically ablate all CySCs in the adult testis. We used the GAL4-UAS system to conditionally express the pro-apoptotic gene grim in all CySCs and cyst cells associated with spermatogonia (early cyst cells) (Fig. 1B). At the permissive temperature of 18˚C, testes were phenotypically wild-type (Fig. 1C, D); by contrast, after shifting flies to the restrictive temperature of 31°C for two days, 100% of testes completely lacked CySCs and early cyst cells, as shown by immunostaining for the CySC and early cyst cell nuclear marker Traffic jam (Tj; Fig. 1E) (Lasko and Ashburner, 1990; Li et al., 2003) and the CySC nuclear marker Zinc finger homeodomain 1 (Zfh1; Fig. 1F, Table S1a) (Leatherman and DiNardo, 2008). As expected, cells that did not express ectopic grim were still present in all testes after two days, including hub cells, outlined by the adhesion protein Cadherin-N (CadN; Fig. 1F) (Sinden et al., 2012); late cyst cells, which surround spermatocytes and express the nuclear marker Eyes absent (Eya; Fig. S1a) (Fabrizio et al., 2003); and germ cells (GSCs, spermatogonia, and spermatocytes), which express the germline marker Vasa (Fig. S1b). Furthermore, every cell that remained outside the hub expressed either germ cell or late cyst cell markers; therefore, CySCs and early cyst cells are being lost, not simply turning off markers. Consistent with this finding, ectopic grim expression in the CySC lineage induced apoptosis in CySCs but not in hub cells or GSCs (Fig. S1c, Table S1b). Since CySC-to-hub cell conversion was reported to occur with aging (Voog et al., 2008) but contradictory results were also reported (DiNardo et al., 2011), we asked if any CySCs escape ablation by becoming hub cells. First, we fed flies the thymidine analog ethynyl deoxyuridine (EdU) to label cells undergoing DNA replication. We dissected half the flies after 72 hours of labeling and found that EdU was incorporated into their germ cells and CySC lineage cells but was not detected in the hub (Fig. S1d). In 75% of testes (n = 18/24), 100% of CySCs were labeled with EdU; in the remaining testes (n = 6/24), all but 1 or 2 CySCs were labeled. We then shifted the remaining flies to 31°C for 2 days to ablate CySCs. EdU was not detected in the hub in any testis after ablation, although it was still detected in germ cells (n = 25 testes; Fig. S1d). Therefore, CySCs marked by EdU did not enter the hub upon CySC ablation. We conclude that ectopic expression of grim in CySCs and early cyst cells for 2 days autonomously induces cell death of all CySCs and early cyst cells; we refer to these conditions as “CySC ablation”.
CySCs are regenerated after ablation
To determine the effects of CySC ablation on the remaining cells in the testis, we returned flies lacking CySCs to the permissive temperature (18°C) and allowed them to recover for two weeks. As expected from previous findings (Lim and Fuller, 2013), 35% of testes (n = 178/506) were “empty” except for the hub, or the hub and early germ cells (not shown). Unexpectedly, 65% of testes (n = 328/506) appeared strikingly similar to wild-type testes: they retained a hub and germ cells but also regained a large population of Tj-positive somatic cells intermingled with germ cells (Fig. 1G). Staining for Zfh1 indicated that CySCs had returned (Fig. 1H). To determine if the new somatic cells were functional, we assayed for the presence of spermatocytes, which cannot form in the absence of cyst cell-derived signals (Lim and Fuller, 2013; Zoller and Schulz, 2012). Although spermatocytes remained immediately after CySC ablation, they were gone from most testes by one week of recovery, as expected after a lapse in cyst cell production. By two weeks of recovery, however, spermatocytes reappeared in most testes that regained CySCs (Fig. S1b, panels J–M) and were associated with Eya-positive cyst cells (Fig. S1a, panel C); this finding indicates that the regenerated CySCs produce cyst cells that support normal germ cell differentiation. We conclude that when all CySCs are ablated, other cells are stimulated to produce new, functional CySCs.
Hub cells exit quiescence in response to CySC ablation
We next asked which cells were giving rise to new CySCs. After CySC ablation, two types of somatic cells remained in all testes: hub cells and Eya-positive cyst cells. As a first step toward determining if one or both of these non-mitotic cell types could give rise to CySCs, we asked if either cell type re-enters the cell cycle after CySC ablation. In vivo incorporation of EdU revealed that hub cells in 3% of testes (n = 3/97) began traversing S-phase within the first five hours of recovery from CySC ablation (Fig. 2A); this increased to 28% of testes (n = 36/128) after 24 hours of labeling (Fig. 2B). By contrast, EdU incorporation was not detected in Eya-positive cyst cells (n = 128 testes, Fig. 2B). Similar results were obtained using the thymidine analog bromodeoxyuridine (BrdU; Fig. S2). Immunostaining testes for the mitotic markers Anillin (Fig. S2) (Field and Alberts, 1995) or Phosphohistone H3 (PH3; Fig. 2C) indicated that hub cells entered mitosis by 24 hours of recovery and remained mitotically active for up to 6 days before returning to quiescence by day 7 (Table S2a). Mitotic hub cells were never detected in control testes under the same environmental conditions or in testes expressing ectopic grim specifically in hub cells (n = 74 and 95 testes, respectively); therefore, loss of hub cell quiescence is not a non-specific response to the conditions used to induce ablation. We conclude that hub cells, but not Eya-positive cyst cells, transiently exit quiescence specifically in response to CySC ablation. This finding led us to speculate that hub cells could be the source of new CySCs; however, it does not rule out a requirement for Eya-positive cells or germ cells in CySC regeneration.
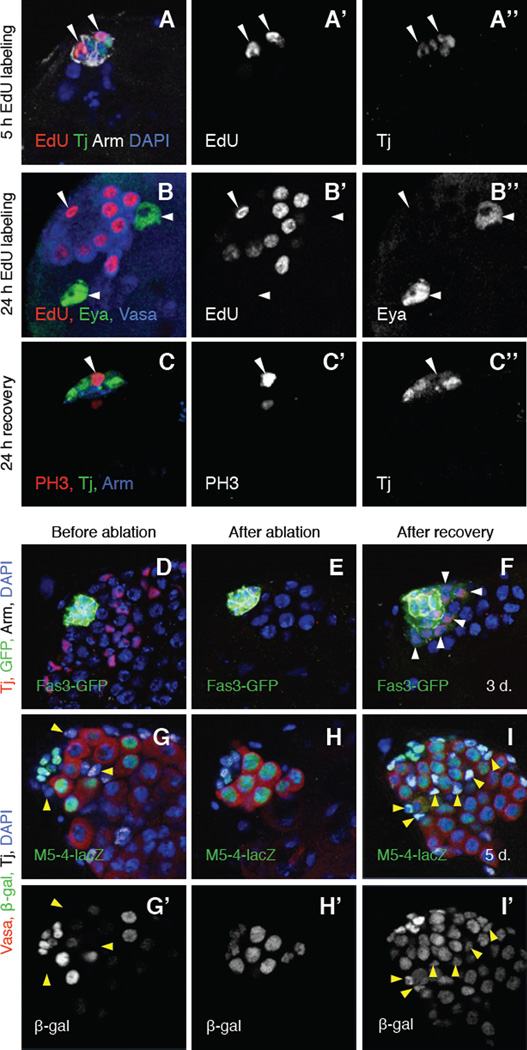
Figure 2. After complete genetic ablation of CySCs, hub cells exit quiescence and convert into CySC-like cells.
(A–I) Single confocal sections through the apex of c587-Gal4>grim testes. Nuclei are counterstained with DAPI (A, D–I, blue). (A–A”) Testis labeled with S-phase marker EdU (red and A’), anti-Tj (green and A”; hub cells) and anti-Arm (white; hub cells). After CySC ablation and 5 hours of recovery with EdU labeling, some hub cells incorporate EdU, indicating that they are no longer quiescent (arrowheads). (B-B”) Testis labeled with EdU (red and B’), anti-Eya (green and B”; late cyst cells), and Vasa (blue; germ cells). After CySC ablation and 24 hours of recovery with EdU labeling, EdU is incorporated into some somatic cells in or adjacent to the hub (one hub cell shown, arrowhead) and into some germ cells (Vasa-positive) but not into any Eya-positive cyst cells (two shown, arrows). (C–C”) Testis labeled with the mitotic marker anti-PH3 (red and C), anti-Tj (green and C”), and anti-Arm (blue). After CySC ablation and 24 hours of recovery, some hub cells express PH3 (arrowhead), indicating that they have entered mitosis. Tj disperses throughout the cytoplasm of mitotic cells. (D–F) Testes immunostained with anti-Tj (red), anti-GFP (green, alone in insets), and anti-Arm (white). GFP expression is driven by the Fas3 promoter. (D) Before and (E) after ablation, GFP is expressed throughout the hub but not in any cells outside the hub. (F) After 3 days of recovery, GFP is found in the hub and in regenerating CySCs (Tj -positive/Arm-negative cells outside the hub; arrows) in 81% of testes (n = 22/27). (G–I) Testes immunostained with anti-Vasa (red), anti-β-Gal (green and G’-I’), and anti-Tj (white). β-Gal expression is driven by M5-4-lacZ. (G–G’) Before and (H–H’) after ablation, M5-4-lacZ marks hub cells and early germ cells but not CySCs or cyst cells (arrows). (I–I’) After 5 days of recovery, β-Gal is found in hub cells, germ cells, and in regenerating CySCs and early cyst cells (Tj-positive/Arm-negative cells outside the hub; arrows). This is likely to reflect continued transcription, rather than perdurance, of the marker. Scale bars, 20µm. See also Figure S2 and Tables S2 and S3.
We next asked if changes in gene expression accompany the loss of hub cell quiescence after CySC ablation. The adhesion protein Armadillo/β-catenin (Arm) is highly enriched on the surface of hub cells in wild-type testes (Yamashita et al., 2003); after CySC ablation Arm was down-regulated in some hub cells (Fig. S2), suggesting that they delaminate from the hub. The hub-specific markers Signal transducer and activator of transcription-GFP (Stat-GFP) (Bach et al., 2007) and hedgehog-lacZ (hh-lacZ) (Forbes et al., 1996) were also transiently down-regulated in hub cells following CySC ablation (Fig. S2), suggesting that changes in niche signals accompany the loss of hub cell quiescence. Furthermore, during recovery from CySC ablation, hub cell markers transiently appeared in the new CySC lineage cells. For example, Fasciclin 3-GFP (Fas3-GFP) was co-expressed with Tj in cells outside the hub after three days of recovery (Fig. 2D–F). Similarly, the enhancer trap M5-4, which marks the hub and early germ cells (Tran et al., 2000), became expressed in all Tj-positive cells in all testes that regained CySCs at five days of recovery (Fig. 2G–I, Table S2ba). By two weeks of recovery, both Fas3-GFP and M5-4 became restricted to the hub again (Fig. 4A, G). Since M5-4 and Tj co-expressing cells were never found in control testes under the same environmental conditions (Table S2b), the temperature shift used for CySC ablation does not induce expression of hub markers in the CySC lineage. We conclude that CySC ablation triggers transient molecular changes in hub cells and regenerating CySCs. These changes, together with the ability of CySCs to be replenished after complete ablation and the ability of hub cells to exit quiescence, led us to hypothesize that hub cells give rise to new CySCs after CySC ablation. Because CySCs arising via conversion transiently co-express markers that are never co-expressed in control testes, we propose that new CySCs may be in an intermediate state that is resolved once the normal balance of cells and signals in the niche is restored.
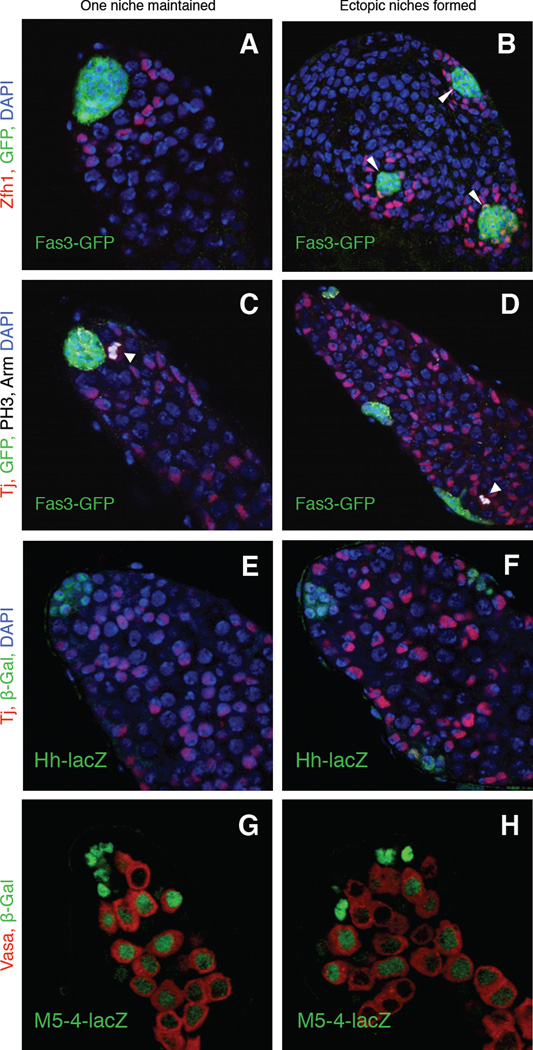
Figure 4. Hub cell conversion to CySCs promotes the formation of ectopic functional stem cell niches.
(A–H) Single confocal sections through the apex of testes immunostained for three different hub markers after CySC ablation and two weeks of recovery. Nuclei are counterstained with DAPI (A–F, blue). (A–D) c587-Gal4>grim testes containing the hub marker Fas3-GFP. (A–B) Testes immunostained with anti-Zfh1 (red; CySCs and their immediate daughters) and anti-GFP (hubs, green). Testes regaining CySCs contain either (A) single (n-= 21/36 testes) or (B) multiple hubs (arrowheads) (n-= 15/36 testes) surrounded by a rosette of CySCs (red). (C–D) Testes immunostained with anti-Tj (red; CySCs and early cyst cells), anti-GFP (green; hub cells), anti-PH3 (white; mitotic cells), and anti-Arm (white; hub cells). Mitotic CySCs (arrows) can be found near (C) single or (D) ectopic hubs (detected in 50/91 and 41/91 testes, respectively). (E–F) c587-Gal4>grim testes containing the hub marker hh-lacZ immunostained with anti-Tj (red) and anti-β-Gal (green, hub cells). In testes with (E) single (n=13/17 testes) or (F) multiple hubs (n=4/17), all hubs (outlined) express hh-lacZ. (G–H) c587-Gal4>grim testes containing the marker M5-4-lacZ immunostained with anti-Vasa (red; germ cells) and anti-β-Gal (M5-4-lacZ, green, hub cells and early germ cells). In testes with (G) single (n=20/40 testes) or (H) multiple hubs (n=20/40 testes), all hubs (outlined) express M5-4-lacZ. Scale bars, 20 µm. See also Figure S3 and Table S5.
Forced expression of CyclinD-Cdk4 causes hub cells to convert into CySCs
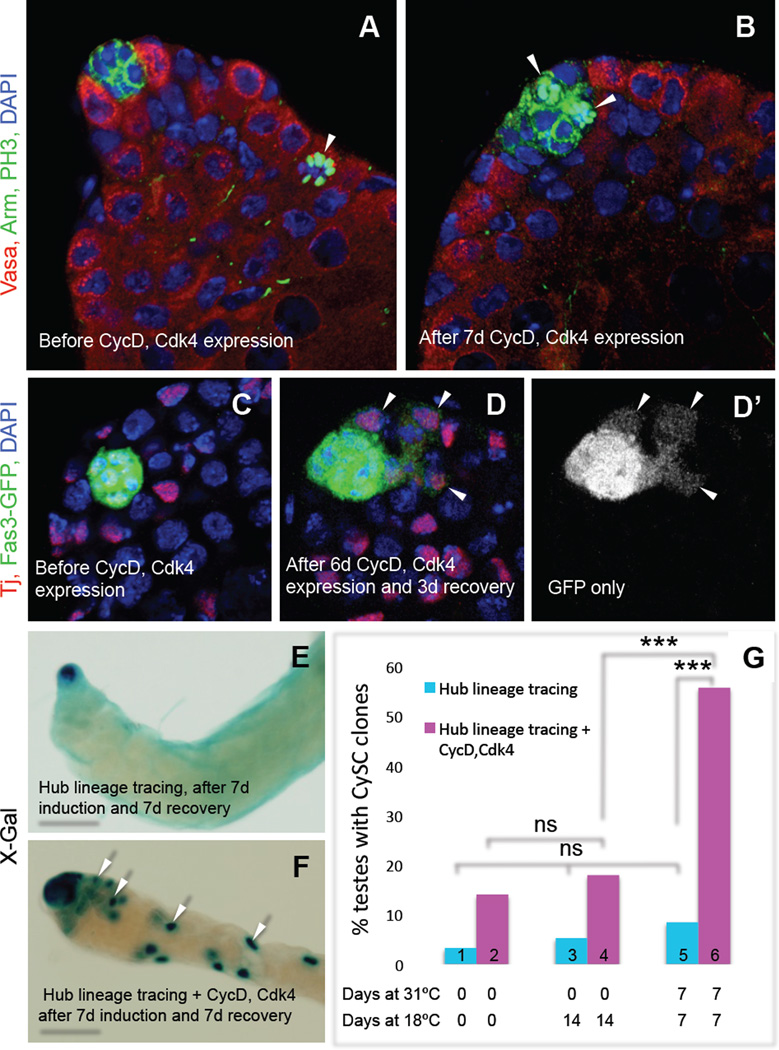
We hypothesized that forcing hub cells to re-enter the cell cycle could be sufficient to trigger their conversion to CySCs in an otherwise wild-type adult testis. Ectopically expressing Cyclin D and Cdk4 (Buttitta et al., 2007) in hub cells caused them to re-enter the cell cycle; hub cells from uninduced controls remained quiescent (Fig. 3A, B). As with CySC ablation, testes with ectopic Cyclin D-Cdk4 expression in hub cells acquired cells near the hub that were morphologically indistinguishable from CySCs but expressed the hub marker Fas3-GFP (Fig. 3C, D). To confirm that these CySCs were derived from hub cells, we used genetic lineage tracing to permanently mark hub cells in adult flies and assayed their ability to produce marked CySC lineage cells (CySC “clones”) following ectopic Cyclin D-Cdk4 expression in the hub. Significantly more testes had CySC clones than did testes from control flies (Fig. 3E–G, Table S4). We conclude that forced re-entry of hub cells into the cell cycle is sufficient to promote their conversion into functional CySCs.
Figure 3. Ectopic CycD-Cdk4 expression in the hub promotes hub cell proliferation and conversion to CySCs.
(A–D) Single confocal sections through the apex of testes expressing CycD and Cdk4 in hub cells, immunostained as indicated. Nuclei are counterstained with DAPI (blue). (A –B) E132-Gal4>CycD, Cdk4 testes immunostained with anti-Vasa (red; germ cells), anti-Arm (green at cell cortex; hub cells), and anti-PH3 (green; mitotic nuclei). (A) Before CycD-Cdk4 induction (18°C), PH3-positive cells (arrow) are found outside but never inside the hub (n = 127 testes). (B) After CycD-Cdk4 expression (7 d, 31°C), 42% of testes (n = 35/83) contain PH3-positive hub cells (two shown, arrowheads). (C–D) E132-Gal4>CycD, Cdk4 testes co-expressing Fas3-GFP (marking hub cells), immunostained with anti-Tj (red; hub cells, CySCs and early cyst cells) and anti-GFP (green). (C) Before CycD-Cdk4 expression (18°C), GFP expression is restricted to the hub. (D-D’) After CycD-Cdk4 expression (6 d, 31°C) and recovery (3 d, 18°C), GFP is observed in Tj-positive cells outside the hub (arrows) in 23% of testes (n = 7/30). (E, F) X-gal staining revealing lineage traced hub cells (outlined) in the absence or presence of ectopic CycD-Cdk4. Testes contain either (E) marked hubs (example is from a control male, genotype: E132-gal4;;UAS-Flp/Act5c>Stop>lacZ), or (F) marked hubs and marked CySCs and their descendants (F, arrows), referred to herein as “CySC clones” (example is from an experimental male, genotype: E132-gal4; UAS-CycD, UAS-Cdk4; UAS-Flp/Act5c>Stop>lacZ). (G) Bar graph showing that flies expressing CycD-Cdk4 in hub cells have significantly more CySC clones than age-matched uninduced flies of the same genotype (bars 4 vs. 6), and than flies lacking ectopic CycD-Cdk4 that are processed in parallel (bars 5 vs. 6). The low percentage of CySC clones in testes from newly eclosed males represents background levels of labeling; this does not change significantly over time (bars 1,3,5). ns, not significant. *** P value < 0.0001 (two-tailed Fisher’s exact test). Scale bars, 20 µm (A–D) or 100 µm (E–F). See also Table S4.
Hub cells do not convert after partial loss of CySCs
To determine if hub cell conversion occurs when CySCs are reduced in number but not completely eliminated, we permanently marked hub cells in adult flies and assayed their ability to produce marked CySC clones after nutritional deprivation. Although subjecting flies to protein starvation and re-feeding causes CySCs to be reduced by about half and then restored (McLeod et al., 2010; Roth et al., 2012; Sheng and Matunis, 2011), we did not see CySC clones above background levels under these conditions (Table S3a). Thus, CySCs lost via nutritional deprivation are not replaced by hub cell conversion. In addition, hub cell conversion did not replace CySCs lost with age in wild-type adults (Table S3b). Consistent with these results, when we ablated CySCs using milder conditions, which removed all CySCs from some but not all testes, we found that hub cells converted to CySCs in some but not all testes, as revealed by the presence of M5-4 and Tj co-expressing cells (Table S2b). Taken together, these results suggest that extrinsic signals from CySCs and early cyst cells may contribute to the prevention of hub cell conversion. If so, these signals are likely to be overriden by forced re-entry of hub cells into the cell cycle.
Ectopic niches form as a consequence of hub cell conversion
The observation that CySCs generated via hub cell conversion differ from normal CySCs by transiently expressing hub cell markers (Fig. 2F, I) prompted us to look more closely at testes recovering from CySC ablation. Surprisingly, after two weeks of recovery, 42% of testes (n = 60/144) contained an average of 2–3 ectopic hubs, as revealed by the expression of three different hub markers (Fig. 4). All hubs were located near the basement membrane and were sometimes comparable in size and appearance, but we considered the most apical hub to be the original hub based on its location. Both apical and ectopic hubs were surrounded by Zfh1-postive cells (Fig. 4B) and Vasa-positive cells with single round fusomes (Fig. S3a, panel A); moreover, putative CySCs and GSCs adjacent to both apical and ectopic hubs expressed the stem cell marker Stat92E (Fig. S3a, panels D, E) and the mitotic marker PH3 (Fig. 4D, Fig. S3a, panels B, C). These results suggest that both apical and ectopic hubs maintain functional CySCs and GSCs. Ectopic Cyclin D-Cdk4 expression in hub cells was also sufficient to induce the formation of ectopic hubs surrounded by putative GSCs and CySCs (Fig. S3b, Table S5). Ectopic hubs may arise from fission of enlarged hubs, since irregular and dumb-bell shaped hubs were observed (Fig. S3); they might also arise from incompletely reprogrammed CySCs that coalesce and revert to hub cells. We conclude that both CySC ablation and intrinsic re-activation of the cell cycle in hub cells are sufficient to prompt hub cell conversion to CySCs, but an unexpected consequence is the formation of ectopic niches containing mitotic stem cells.
Discussion
We have uncovered a novel mechanism for regenerating stem cells after tissue damage: conversion of quiescent niche cells into somatic stem cells. The ability of hub cells to convert to CySCs is intriguing in light of the close relationship between these cells. which arise from a common pool of precursors in the embryo (DiNardo et al., 2011). In the posterior region of the gonad, signals from germ cells repress hub cell formation and allow CySCs to develop; in the absence of these signals, excess hub cells are formed (Kitadate et al., 2007; Kitadate and Kobayashi, 2010). Excess hub cells are also formed in embryos mutant for the essential CySC factor lines. CySCs depleted of lines in adult flies, however, only partially adopt a hub cell fate. Therefore, other factors must be acting in adults to maintain the distinction between CySCs and hub cells. Identifying these factors will allow us to gain insight into niche formation during development and into the mechanisms promoting or preventing the formation of new niches in adult tissue.
The regulation of quiescence in vivo is not well understood in most tissues, but an emerging concept is that this state must be actively maintained over time (O'Farrell, 2011). The reversal of quiescence is a key feature of many adult stem cells, and our work implicates stem cells themselves as a likely source of signals promoting quiescence in neighboring cells. Our preliminary data indicate that overexpression of Cyclin E-E2F in hub cells recapitulates our Cyclin D-Cdk4 results, supporting the hypothesis that oncogenic mutations in quiescent niche cells can cause niches to expand uncontrollably. Since cyclin overexpression is a common feature of many human cancers, the ability of quiescent niche cells to convert into mis-regulated stem cells after tissue damage may be broadly relevant to human cancers.
Experimental Procedures
Transgene induction
Flies were maintained on standard yeast medium at 18°C. To induce grim expression in CySCs and early cyst cells, males with the genotype c587-Gal4; UAS-grim/+; tub-Gal80ts/+ or w; Tj-Gal4/UAS-grim; Gal80ts/+ were shifted to 31°C for 2 days. To induce grim expression in hub cells, E132-Gal4; UAS-grim/+; tub-Gal80ts/+ males were shifted to 31°C for 2 days and returned to 18°C for one day. To induce CycD-Cdk4 expression in hub cells, E132-Gal4; UAS-CycD, UAS-Cdk4/+; tub-Gal80ts/+ males were shifted to 31°C for 7 days. E132-Gal4; UAS-CycD, UAS-Cdk4/Fas3-GFP; tub-Gal80ts/+ males were shifted for 6 days and returned to 18°C for 3 days (for finding transient GFP in new CySCs) or were shifted for 2 weeks and returned to 18°C for 2 weeks (for finding ectopic hubs).
In vivo BrdU and EdU labeling
To determine if hub cells and/or Eya-positive cyst cells incorporate S-phase markers after CySC ablation, CySCs were ablated, and then flies were fed 10 mM EdU or 2.5 mM BrdU in apple juice for 24 hours at 18°C before dissection. The testis apex was analyzed by serial confocal microscopy and the rest of the testis was scored via epifluorescence, revealing an average of 4 Eya-positive cells per experimental testis. To determine if CySCs escape ablation by entering the hub during grim induction, flies were fed EdU at 18°C for 72 hours; half were dissected immediately, while the rest were shifted to 31°C for 2 days to ablate CySCs and then dissected.
Aging and nutritional deprivation
For aging experiments, males were aged at 25°C on standard yeast medium. For nutritional deprivation experiments, males were placed in vials containing 10% sucrose/1% agar food for 3 weeks as described (McLeod et al., 2010) and then fed standard yeast medium for 5 days.
See Supplemental Experimental Procedures for fly stocks, immunofluorescence microscopy, apoptosis and β-galactosidase detection, drug feeding, and lineage tracing of hub cells.
Supplementary Material
Highlights.
Somatic cyst stem cells (CySCs) in adult Drosophila testes can be genetically ablated.
CySC ablation causes quiescent hub cells to convert into new CySCs.
Forced expression of Cyclin D-Cdk4 in the hub causes hub cell conversion.
Multiple ectopic niches form as a result of hub cell conversion.
Acknowledgments
We thank J. Abrams, S. DiNardo, D. Drummond-Barbosa, B. Edgar, D. McKearin, B. Ohlstein, A. Spradling, and the Bloomington Drosophila Stock Center for flies; E. Bach, C. Field, D. Godt, R. Lehmann, and the Developmental Studies Hybridoma Bank for antibodies; L. Greenspan, M. Matunis, R. Stine, and Q. Ma for comments; and M. Fuller and J. Lim for sharing data prior to publication. Supported by NIH grants RO1HD040307 and RO1HD052937 (E.M.).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Author contributions
P.H., M.C., and E.M. designed the research, analyzed the data, and wrote the paper; P.H. and M.C. performed the experiments.
The authors have no conflicts of interest.
References
- Bach EA, Ekas LA, Ayala-Camargo A, Flaherty MS, Lee H, Perrimon N, Baeg GH. GFP reporters detect the activation of the Drosophila JAK/STAT pathway in vivo. Gene Expr Patterns. 2007;7:323–331. doi: 10.1016/j.modgep.2006.08.003. [DOI] [PubMed] [Google Scholar]
- Brawley C, Matunis E. Regeneration of male germline stem cells by spermatogonial dedifferentiation in vivo. Science. 2004;304:1331–1334. doi: 10.1126/science.1097676. [DOI] [PubMed] [Google Scholar]
- Buttitta LA, Katzaroff AJ, Perez CL, de la Cruz A, Edgar BA. A double-assurance mechanism controls cell cycle exit upon terminal differentiation in Drosophila. Dev Cell. 2007;12:631–643. doi: 10.1016/j.devcel.2007.02.020. [DOI] [PubMed] [Google Scholar]
- Cheng J, Turkel N, Hemati N, Fuller MT, Hunt AJ, Yamashita YM. Centrosome misorientation reduces stem cell division during ageing. Nature. 2008;456:599–604. doi: 10.1038/nature07386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Cuevas M, Matunis EL. The stem cell niche: lessons from the Drosophila testis. Development. 2011;138:2861–2869. doi: 10.1242/dev.056242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DiNardo S, Okegbe T, Wingert L, Freilich S, Terry N. lines and bowl affect the specification of cyst stem cells and niche cells in the Drosophila testis. Development. 2011;138:1687–96. doi: 10.1242/dev.057364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fabrizio JJ, Boyle M, DiNardo S. A somatic role for eyes absent (eya) and sine oculis (so) in Drosophila spermatocyte development. Dev Biol. 2003;258:117–128. doi: 10.1016/s0012-1606(03)00127-1. [DOI] [PubMed] [Google Scholar]
- Field CM, Alberts BM. Anillin, a contractile ring protein that cycles from the nucleus to the cell cortex. J Cell Biol. 1995;131:165–178. doi: 10.1083/jcb.131.1.165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forbes AJ, Lin H, Ingham PW, Spradling AC. hedgehog is required for the proliferation and specification of ovarian somatic cells prior to egg chamber formation in Drosophila. Development. 1996;122:1125–1135. doi: 10.1242/dev.122.4.1125. [DOI] [PubMed] [Google Scholar]
- Hsu YC, Fuchs E. A family business: stem cell progeny join the niche to regulate homeostasis. Nat Rev Mol Cell Biol. 2012;13:103–114. doi: 10.1038/nrm3272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kitadate Y, Kobayashi S. Notch and Egfr signaling act antagonistically to regulate germ-line stem cell niche formation in Drosophila male embryonic gonads. Proc Natl Acad Sci U S A. 2010;107:14241–14246. doi: 10.1073/pnas.1003462107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kitadate Y, Shigenobu S, Arita K, Kobayashi S. Boss/Sev signaling from germline to soma restricts germline-stem-cell-niche formation in the anterior region of Drosophila male gonads. Dev Cell. 2007;13:151–159. doi: 10.1016/j.devcel.2007.05.001. [DOI] [PubMed] [Google Scholar]
- Lasko PF, Ashburner M. Posterior localization of vasa protein correlates with, but is not sufficient for, pole cell development. Genes Dev. 1990;4:905–921. doi: 10.1101/gad.4.6.905. [DOI] [PubMed] [Google Scholar]
- Leatherman JL, DiNardo S. Zfh-1 controls somatic stem cell self-renewal in the Drosophila testis and nonautonomously influences germline stem cell self-renewal. Cell Stem Cell. 2008;3:44–54. doi: 10.1016/j.stem.2008.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leatherman JL, DiNardo S. Germline self-renewal requires cyst stem cells and stat regulates niche adhesion in Drosophila testes. Nat Cell Biol. 2010;12:806–811. doi: 10.1038/ncb2086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li MA, Alls JD, Avancini RM, Koo K, Godt D. The large Maf factor Traffic Jam controls gonad morphogenesis in Drosophila. Nat Cell Biol. 2003;5:994–1000. doi: 10.1038/ncb1058. [DOI] [PubMed] [Google Scholar]
- Lim JG, Fuller MT. Somatic cell lineage is required for differentiation and not maintenance of germline stem cells in Drosophila testes. Proc Natl Acad Sci U S A. 2013;109:18477–18481. doi: 10.1073/pnas.1215516109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLeod CJ, Wang L, Wong C, Jones DL. Stem cell dynamics in response to nutrient availability. Curr Biol. 2010;20:2100–2105. doi: 10.1016/j.cub.2010.10.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Farrell PH. Quiescence: early evolutionary origins and universality do not imply uniformity. Philos Trans R Soc Lond B Biol Sci. 2011;366:3498–3507. doi: 10.1098/rstb.2011.0079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roth TM, Chiang CY, Inaba M, Yuan H, Salzmann V, Roth CE, Yamashita YM. Centrosome misorientation mediates slowing of the cell cycle under limited nutrient conditions in Drosophila male germline stem cells. Mol Biol Cell. 2012;23:1524–1532. doi: 10.1091/mbc.E11-12-0999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheng XR, Brawley CM, Matunis EL. Dedifferentiating spermatogonia outcompete somatic stem cells for niche occupancy in the Drosophila testis. Cell Stem Cell. 2009;5:191–203. doi: 10.1016/j.stem.2009.05.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheng XR, Matunis E. Live imaging of the Drosophila spermatogonial stem cell niche reveals novel mechanisms regulating germline stem cell output. Development. 2011;138:3367–3376. doi: 10.1242/dev.065797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sinden D, Badgett M, Fry J, Jones T, Palmen R, Sheng X, Simmons A, Matunis E, Wawersik M. Jak-STAT regulation of cyst stem cell development in the Drosophila testis. Dev Biol. 2012;372:5–16. doi: 10.1016/j.ydbio.2012.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tran J, Brenner TJ, DiNardo S. Somatic control over the germline stem cell lineage during Drosophila spermatogenesis. Nature. 2000;407:754–757. doi: 10.1038/35037613. [DOI] [PubMed] [Google Scholar]
- Visvader JE, Lindeman GJ. Cancer stem cells: current status and evolving complexities. Cell Stem Cell. 2012;10:717–728. doi: 10.1016/j.stem.2012.05.007. [DOI] [PubMed] [Google Scholar]
- Voog J, D'Alterio C, Jones DL. Multipotent somatic stem cells contribute to the stem cell niche in the Drosophila testis. Nature. 2008;454:1132–1136. doi: 10.1038/nature07173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamashita YM, Jones DL, Fuller MT. Orientation of asymmetric stem cell division by the APC tumor suppressor and centrosome. Science. 2003;301:1547–1550. doi: 10.1126/science.1087795. [DOI] [PubMed] [Google Scholar]
- Zoller R, Schulz C. The Drosophila cyst stem cell lineage: Partners behind the scenes? Spermatogenesis. 2012;2:145–157. doi: 10.4161/spmg.21380. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.