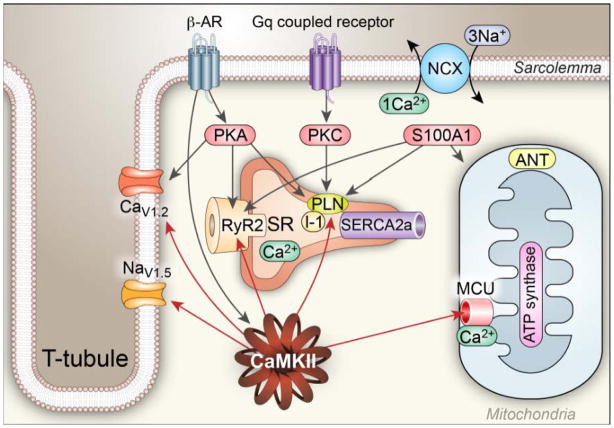
Figure 2. Regulation of [Ca2+]i homeostasis by Ca2+ binding proteins and kinases.
Regulation of Ca2+ homeostasis involves a multitude of Ca2+ binding proteins and enzymes, including CaMKII, PKC, PKA and S100A1: (1). CaMKII catalyzes phosphorylation of voltage-gated Ca2+ channels (mostly CaV1.2 in ventricle) to increase Ca2+ entry, RyR2 to increase Ca2+ release, voltage-gated Na+ channels (mostly NaV1.5 in ventricle) to increase subsarcolemmal [Na+]i,, which decreases the driving force for Ca2+ extrusion by the Na+/Ca2+ exchanger (NCX), and PLN to reduce the inhibitory activity of PLN on SERCA2a. In general, the increased phosphorylation of these proteins by CaMKII increases Ca2+ influx, and storage by the SR, which leads to increased systolic [Ca2+]i and increased rate and magnitude of force (pressure) generation and improved lusitropy. (2) PKA is activated by β–AR agonists and catalyzes phosphorylation of the same Ca2+ regulatory proteins modified by CaMKII, but at different amino acids. (3) Classical PKC isoforms are activated downstream to a variety of G protein coupled receptors and are activated by increased [Ca2+]i, leading to decreased activity SERCA2 by phosphorylating inhibitor 1 (I-1) resulting in PLN dephosphorylation, reducing SR Ca2+ load and Ca2+ release, causing reduced contractility. (4) S100A1 interacts with the SERCA2a/PLN complex in a Ca2+-dependent manner to augment SR Ca2+ uptake and increase SR Ca2+ content. S100A1 also directly regulates RyR2 function, stimulates ATP synthase activity and promotes the adenosine nucleotide translocator (ANT) function to increase ATP synthesis and mitochondrial ATP efflux in cardiomyocytes.