Abstract
Alterations in lipid metabolism within the heart may have a causal role in the establishment of diabetic cardiomyopathy; however, this remains equivocal. Therefore, in the current study we determined cardiac mitochondrial bioenergetics in ZDF rats before overt type 2 diabetes and diabetic cardiomyopathy developed. In addition, we utilized resveratrol, a compound previously shown to improve, prevent or reverse cardiac dysfunction in high-fat-fed rodents, as a tool to potentially recover dysfunctions within mitochondria. Fasting blood glucose and invasive left ventricular haemodynamic analysis confirmed the absence of type 2 diabetes and diabetic cardiomyopathy. However, fibrosis was already increased (P < 0.05) ∼70% in ZDF rats at this early stage in disease progression. Assessments of mitochondrial ADP and pyruvate respiratory kinetics in permeabilized fibres from the left ventricle revealed normal electron transport chain function and content. In contrast, the apparent Km to palmitoyl-CoA (P-CoA) was increased (P < 0.05) ∼60%, which was associated with an accumulation of intracellular triacylgycerol, diacylglycerol and ceramide species. In addition, the capacity for mitochondrial reactive oxygen species emission was increased (P < 0.05) ∼3-fold in ZDF rats. The provision of resveratrol reduced fibrosis, P-CoA respiratory sensitivity, reactive lipid accumulation and mitochondrial reactive oxygen species emission rates. Altogether the current data support the supposition that a chronic dysfunction within mitochondrial lipid-supported bioenergetics contributes to the development of diabetic cardiomyopathy, as this was present before overt diabetes or cardiac dysfunction. In addition, we show that resveratrol supplementation prevents these changes, supporting the belief that resveratrol is a potent therapeutic approach for preventing diabetic cardiomyopathy.
Key points
Dysfunctional mitochondrial respiration may contribute to the establishment of diabetic cardiomyopathy, but this remains controversial; resveratrol, a polyphenol compound, has been shown to recover heart contractile function in rodent models of high-fat-diet-induced cardiac dysfunction.
Therefore, we studied mitochondrial respiratory kinetic function in ZDF rats before overt diabetes and cardiac dysfunction manifested, and determined the efficacy of resveratrol to recover potential derangements in mitochondrial bioenergetics.
We show that the electron transport chain functions normally in ZDF rats, as pyruvate and ADP respiratory kinetics were normal.
In contrast, in ZDF rats, we show impairments in the sensitivity of mitochondria to lipids (palmitoyl-CoA) as well as the accumulation of reactive lipids and increased mitochondrial reactive oxygen species (ROS) emission rates.
Supplementation with resveratrol improved palmitoyl-CoA respiratory kinetics and reactive lipid profiles, and normalized mitochondrial ROS emission rates.
Introduction
Obesity, insulin resistance and dyslipidaemia are strong risk factors for the development of cardiovascular disease and the pathogenesis of atherosclerosis. While dyslipidaemia and elevated plasma lipid profiles have well-defined roles in the generation of atherosclerotic plaque formation, recent evidence also suggests that lipid accumulation within cardiac muscle may participate in the development of left ventricular dysfunction and diastolic heart failure in type 2 diabetes. Heart disease is a leading cause of death in type 2 diabetic patients, regardless of the presence of coronary artery disease and/or hypertension, a pathology termed diabetic cardiomyopathy (Stanley et al. 1997).
While the underlying cause of diabetic cardiomyopathy is probably multifactorial, one of the proposed mechanisms is impaired rates of ATP production. The heart produces more than 90% of its energy aerobically (Wisneski et al. 1987; Chandler et al. 2003), the majority of which is derived from aerobic lipid metabolism (Ventura-Clapier et al. 2011). Therefore, alterations in lipid metabolism within the heart may have a causal role in the establishment of diabetic cardiomyopathy. In support of this, cardiomyocyte-specific deletion of lipoprotein lipase (LpL) compromises lipid metabolism and the ejection fraction of hearts (Augustus et al. 2006). In addition, acipimox, an anti-lipolytic drug, reduced cardiac work in both healthy control and idiopathic dilated cardiomyopathy patients, supporting the belief that lipid metabolism is required for optimal cardiac performance (Tuunanen et al. 2006). While these studies suggest that increasing free fatty acid delivery to the heart improves contractile performance in some models of heart failure, increased plasma membrane transport of lipids, and the resultant accumulation of reactive lipids, have been strongly associated with diastolic dysfunction, fibrosis, left ventricular hypertrophy, and myocellular death in diabetic cardiomyopathy (Zhou et al. 2000; Finck et al. 2003; Yagyu et al. 2003; Stanley et al. 2005). Clearly, the balance of lipid transport and fatty acid oxidation within the heart is important for cardiac contractile performance.
Reactive lipid accumulation may also result from a dysfunction in mitochondrial fatty acid oxidation. Impaired mitochondrial respiratory function as a result of an attenuation in ADP sensitivity (increased apparent Km for ADP) has been suggested to be a primary cause of pressure-induced diastolic heart failure (Ventura-Clapier et al. 2011). However, ADP sensitivity appears to be normal in the atria of human individuals with type 2 diabetes (Anderson et al. 2009a). Instead, a reduction in mitochondrial fatty acid oxidation, primarily as a result of a repressed peroxisome proliferator-activated receptor gamma coactivator 1-alpha-mediated gene expression has been associated with models of heart failure (Ventura-Clapier et al. 2011). In support of this, permeabilized muscle fibres from the atria of type 2 diabetic humans display repressed maximal palmitoylcarnitine respiration (Anderson et al. 2009a). However, the same research group has also shown in rodents that 12 weeks of a high-fat high-sucrose diet does not reduce palmitoylcarnitine respiration or apparent kinetics in the heart (Fisher-Wellman et al. 2013). Clearly, the role of impaired mitochondrial lipid metabolism in diabetic cardiomyopathy remains equivocal (Belke et al. 2000; Neitzel et al. 2003; Coort et al. 2004; Mazumder et al. 2004; Wang et al. 2005; Bugger et al. 2009). Adding further complexity is the observation that decreasing lipid transport into mitochondria through the administration of etomoxir and/or perhexiline improves cardiac function, even in the diabetic heart (Schmitz et al. 1995; Turcani & Rupp, 1997; Lee et al. 2005; Abozguia et al. 2010). These discrepancies highlight the likelihood that alterations in lipid metabolism could have divergent effects depending on the stage of heart failure, and therefore elucidating potential mitochondrial dysfunctional in lipid metabolism before the establishment of diabetic cardiomyopathy is of paramount importance in understanding the disease progression.
Therefore, in the current study we determined cardiac mitochondrial bioenergetics in Zucker diabetic fatty (ZDF) rats. The ZDF rat represents a modest model for diabetic cardiomyopathy and diastolic dysfunction (Schäfer et al. 2006; Marsh et al. 2007; Baynes & Murray, 2009; Radovits et al. 2009; van den Brom et al. 2009; Daniels et al. 2010, 2012), and we aimed to determine bioenergetics before overt type 2 diabetes and diabetic cardiomyopathy developed. Specifically we completed haemodynamic analysis of the left ventricle, and the Michaelis–Menten kinetic reponses of left ventricular permeabilized muscle fibres to various substrates, including ADP, pyruvate and palmitoyl-CoA (P-CoA) in animals at 11 weeks of age. While the function of the electron transport chain appeared normal, we show that the apparent Km for P-CoA is increased ∼50% in ZDF rats, which was associated with intramyocellular lipid accumulation and an increase in mitochondrial reactive oxygen species (ROS) emission. In addition, administration of resveratrol, a compound previously shown to improve diastolic heart function in type 1 diabetic animals (Delucchi et al. 2012), and prevent or reverse cardiac dysfunction in high-fat-fed rodents (Louis et al. 2012; Qin et al. 2012), normalized P-CoA respiratory kinetics in the ZDF rat, while concomitantly improving intramyocellular lipid profiles and mitochondrial ROS emission rates. The current data strongly suggest that attenuations in mitochondrial respiratory sensitivity to P-CoA precedes the development of left ventricular dysfunction associated with diabetic cardiomyopathy, and that resveratrol treatment is able to normalize mitochondrial bioenergetic responses in pre-diabetic hearts.
Methods
Animals
Male ZDF rats (Charles River, St-Constant, QC, Canada) were housed in individual cages, with a reverse 12 h:12 h light–dark cycle, and were provided with food and water ad libitum. Lean control (LC) rats (n = 13) were fed a standard chow (Purina 5008 diet; Purina, St Louis, MO, USA). Twenty Six ZDF rats (n = 13 each group) were randomly assigned to either a stock diet (ZDF) or a stock diet supplemented with resveratrol (Cayman Chemicals; 200 mg (kg body weight)−1), similar to previous publications (Lagouge et al. 2006) for 6 weeks (Z-Resv). Thereafter rats were anaesthetized with isoflurane and oxygen (2%:98%), left ventricular haemodynamics determined as outlined below, and then the left ventricle excised and either immediately used for bioenergetics assessments, fixed for histology or immediately frozen in liquid nitrogen. This study was approved by the University of Guelph Animal Care Committee, and conforms to the guide for the care and use of laboratory animals published by the US National Institutes of Health.
Measurement of in vivo left ventricular haemodynamics
Rats were anaesthetized with isoflurane and a catheter was inserted through the right carotid artery into the left ventricle. Recordings of haemodynamic signals were collected 15 min later. Maximal left ventricular pressure (LVP max), maximal rates of pressure development (+dP/dt), end-diastolic pressure (EDP) and maximal rates of pressure decline (−dP/dt) were collected.
Histochemical determination of left ventricular fibrosis
Left ventricle was fixed in 10% neutral buffered formalin (VWR, Mississauga, ON, Canada) dehydrated in xylene (Fisher Scientific, Ottawa, ON, Canada) and embedded in paraffin. Five micrometre sections were mounted on charged 1.2 mm Superfrost slides (Fisher Scientific) and stained using picrosirius red. Sections were subsequently imaged using an Olympus FSX 100 light microscope and images were acquired in Cell Sense software (Olympus, Tokyo, Japan). Using standard light microscopy, picrosirius red staining reveals collagen as red and muscle fibres and cytoplasm as yellow. For determination of fibrosis, Cell Sense software was used to threshold images, which isolated total area of red (fibrosis) and total area of yellow (cytoplasm/muscle fibre). Fibrosis was expressed as a percentage of total tissue area. For each animal, fibrosis was determined by averaging five different locations within the left ventricle.
Preparation of permeabilized muscle fibres
The preparation of permeabilized muscle fibre bundles was adopted from prior publications (Perry et al. 2011), as we have previously reported (Smith et al. 2012). Following dissection of the ventricle, fibre bundles (∼2 mg) were separated from the lumen wall in BIOPS buffer containing: CaK2EGTA (2.77 mm), K2EGTA (7.23 mm), Na2ATP (5.77 mm), MgCl2.6H2O (6.56 mm), sodium phosphocreatine (15 mm), imidazole (20 mm), dithiothreitol (0.5 mm) and MES (50 mm). Following separation, fibre bundles were placed in BIOPS containing 40 μg ml−1 saponin, agitated for 30 min and then fibres prepared for respiration were washed in respiration buffer (MiRO5) containing EGTA (0.5 mm), MgCl2.6H2O (3 mm), potassium lactobionate (60 mm), KH2PO4 (10 mm), Hepes (20 mm), sucrose (110 mm) and fatty acid-free bovine serum albumin (1 g l−1). Fibres were left in cold MiRO5 until analysis.
Permeabilized muscle fibre respiration
Mitochondrial respiration was measured in permeabilized fibres by high-resolution respirometry (Oroboros Oxygraph-2k, Innsbruck, Austria) at 37°C and room air saturated oxygen tension in the presence of 25 μm blebbistatin (Perry et al. 2011, 2012). Separate permeabilized fibres from the same animal were used to determine the kinetic properties of ADP, pyruvate and palmitoyl-CoA (P-CoA). ADP (0, 100, 175, 250, 500, 1000, 2000, 4000, 6000 μm)-stimulated respiration was determined in the presence of 10 mm pyruvate and 5 mm malate. Pyruvate (0, 15, 30, 50, 75, 150, 500, 1000 μm)-stimulated respiration was determined in the presence of 5 mm ADP and 5 mm malate, while palmitoyl-CoA (P-CoA; 0, 10, 20, 40, 60, 80, 100, 125, 150, 250 μm)-supported respiration was determined in the presence of 5 mm ADP, 5 mm malate and 2 mm l-carnitine. Exogenous cytochrome c (10 μm) was added at the end of all respiration experiments to ensure outer mitochondrial membrane integrity. The addition of cytochrome c did not significantly elevate the respiration rate in any experiment.
Western blotting
Whole muscle was homogenized in lysis buffer and 5 μg of protein separated by SDS-polyacrylamide gel electrophoresis, transferred to polyvinylidene difluoride membrane, and quantified as previously reported (Smith et al. 2013). The following commercially available antibodies were used to detect α-tubulin (Abcam, Cambridge, MA, USA), oxidative phosphorylation (OXPHOS; Mitosciences), superoxide dismutase 2 (SOD2; Abcam) and catalase (CAT; Abcam) proteins. Bands were visualized using enhanced chemiluminescence (Western Lightning Plus-ECL, PerkinElmer), a FluorChem HD2 Alpha Innotech imager and quantified using the software provided.
Biochemical determination of intramuscular lipid content
Intramuscular levels of triacylglycerol, diacylglycerol and ceramide were measured as we have described previously by gas chromatography (Bonen et al. 2009).
Permeabilized muscle fibre mitochondrial H2O2 emission
Measurement of mitochondrial H2O2 emission was similar to previously described methodology (Anderson et al. 2009b). Briefly, mitochondrial H2O2 emission was determined fluorometrically (Lumina; Thermo Scientific, Fisher) in a constantly stirring cuvette at 37°C (Peltier controlled). Fibres were placed in a cuvette containing Buffer Z (Anderson et al. 2009b) supplemented with 25 μm blebbistatin, 40 U ml−1 of CuZnSOD, 10 μm Amplex Red (Invitrogen) and 0.5 U ml−1 horseradish peroxidase. Mitochondrial H2O2 emission was initiated by the addition of 10 mm succinate. The rate of H2O2 emission was calculated from the slope (fluorescence second−1), after subtracting the background, from a standard curve established with the same reaction conditions and normalized to freeze-dried muscle weight.
Glutathione measurements
Reduced glutathione (GSH) and oxidized glutathione (GSSG) measurements were determined as previously described (Smith et al. 2013). Briefly, four small pieces of the left ventricle were separated while in liquid nitrogen. Two samples (in triplicate wells) were used to determine GSH and two samples (in triplicate wells) were used to determine GSSG in the presence of the scavenger methyl-2-vinylpyridinium triflate (M2VP). Total GSH and GSSG were measured as per manufacturer's instructions (Oxis International, Inc., Beverley Hills, CA, USA).
Statistics
The apparent Km for ADP, glutamate, pyruvate and P-CoA were estimated in independent experiments with Michaelis–Menten kinetics utilizing Prism software (GraphPad Software, Inc., La Jolla, CA, USA), while the maximal respiration (Vmax) was specified as the highest respiration value directly determined, as published previously (Perry et al. 2011). A one-way ANOVA with Newman–Keuls post hoc analysis was used for all comparisons. Statistical significance was accepted with a P value ≤ 0.05, and values represent means and standard error of the mean (SEM).
Results
Resveratrol improves systolic and diastolic function in the heart of ZDF rats
Diastolic dysfunction is a well-characterized phenotype of the ZDF rat (Schäfer et al. 2006; Marsh et al. 2007; Baynes & Murray, 2009; Radovits et al. 2009; van den Brom et al. 2009; Daniels et al. 2010, 2012). Previous reports have assessed cardiac function of ZDF rats at 14 weeks of age or later; however, we aimed to determine mitochondrial bioenergetics in animals before overt type 2 diabetes developed to determine potential mechanisms that subsequently cause diabetic cardiomyopathy. The ZDF rat has a rapid onset of insulin resistance and type 2 diabetes, with average development of type 2 diabetes at 12 weeks of age. At 11 weeks of age only four ZDF animals displayed increased fasting blood glucose (data not shown), confirming the absence of overt type 2 diabetes. Therefore, we assessed cardiac function in 11-week-old rats. Specifically, we characterized heart function using invasive left ventricular haemodynamics to ensure overt diabetic cardiomyopathy had not already developed. While the ZDF rats displayed an increase in end-diastolic pressure (EDP), there were no alterations in maximal left ventricular pressure (LVP max), rates of relaxation (−dP/dt) and force development (Fig. 1). Combined, these data suggest that at 11 weeks of age ZDF animals begin to display the presence of marginal diastolic dysfunction, and therefore represent an ideal model/age to determine associations between changes in mitochondrial bioenergetics and the subsequent establishment of diabetic cardiomyopathy.
Figure 1. Left ventricular pressure characteristics were determined in lean (LC), Zucker diabetic fatty rats (ZDF) and ZDF rats supplemented with resveratrol (Z-Resv).
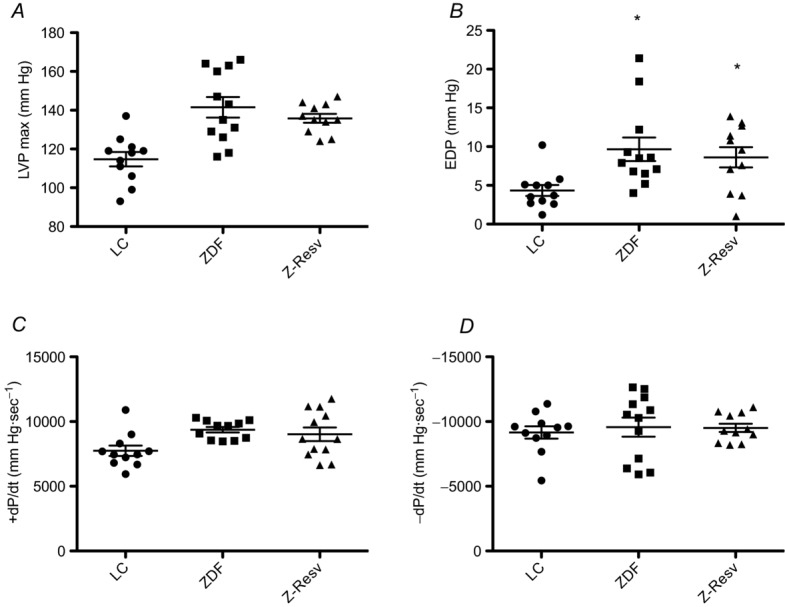
Maximal left ventricular pressure (A; LVP max); end-diastolic pressure (B; EDP); maximal rate of pressure development (C; +dP/dt) and maximal rate of pressure decline (D; −dP/dt) were determined in 11-week-old animals. Scatter plots depict the individual values as well as the means ± SEM. n = 11–12. *Significantly (P < 0.05) different from LC.
Resveratrol has previously been shown to delay mortality, improve ejection fraction, cardiac output, and prevent diastolic dysfunction in models of hypertension as well as type 1 diabetes and obesity (Rimbaud et al. 2011; Delucchi et al. 2012; Louis et al. 2012; Qin et al. 2012; Robich et al. 2012). In skeletal muscle, resveratrol has been shown to alter several aspects of mitochondrial bioenergetics, and therefore we used resveratrol as a potential modality to alter bioenergetics in the hearts of ZDF rats. With respect to haemodynamic analysis, resveratrol did not affect cardiac performance, which is not unexpected given the lack of profound diastolic dysfunction at this early age (Fig. 1). Three main mechanisms have been proposed to cause diabetic cardiomyopathy, including ventricle fibrosis, impaired ATP production and increased redox stress, and therefore we examined all in ZDF rats at 11 weeks of age.
Heart fibrosis is improved by resveratrol supplementation in ZDF rats
Cardiac remodelling, and in particular an increase in cardiac fibrosis, has been affiliated with diastolic dysfunction. In addition, resveratrol has been shown to attenuate fibrosis in a model of type 1 diabetes (Delucchi et al. 2012) and in high-fat-fed mouse model of type 2 diabetes (Qin et al. 2012). We therefore examined myocardial fibrosis to determine if this was increased in ZDF animals or altered following resveratrol supplementation. ZDF rats displayed increased (P < 0.05) fibrosis (Fig. 2A and B), consistent with previous reports (Daniels et al. 2012). However, interestingly in the current study fibrosis was increased ∼65% at 11 weeks of age, comparable to the increase previously reported in 45-week-old ZDF rats (Daniels et al. 2012). It would therefore appear that fibrosis is an early event in the development of diabetic cardiomyopathy in the ZDF rat that is not exacerbated over time. In contrast, fibrosis was decreased ∼30% in ZDF rats supplemented with resveratrol and therefore, compared to lean control rats, cardiac fibrosis was not different in ZDF rats following resveratrol supplementation (Fig. 2A and B).
Figure 2. Left ventricular fibrosis in LC, ZDF and Z-Resv rats.
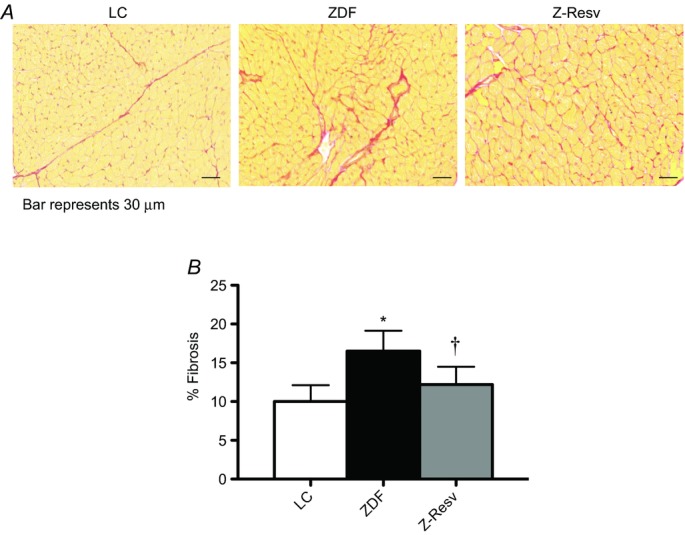
Representative images are shown at ×4.2 magnification, and the black bar represents 30 μm. Values represent means ± SEM. n = 4–5. *Significantly (P < 0.05) different from LC. †Significantly (P < 0.05) different from ZDF.
ADP respiratory sensitivity is impaired in ZDF rats and not recovered by resveratrol
Alterations in mitochondrial energy metabolism have repeatedly been implicated as a cause of diastolic dysfunction (reviewed in Ventura-Clapier et al. 2011). In particular, attenuated mitochondrial ADP-respiratory sensitivity in both the presence and absence of creatine is present in several models of heart failure (reviewed in Ventura-Clapier et al. 2011). Therefore, in the current study we first determined the apparent ADP-respiratory sensitivity in the presence of saturating pyruvate and malate. In all groups a typical Michaelis–Menten curve was observed (Fig. 3A), and therefore the apparent Km and Vmax were determined. However, in contrast to other models of heart failure, neither maximal ADP-stimulated respiration (Fig. 3B) nor the sensitivity to ADP (Fig. 3C) were altered in the ZDF rat or following resveratrol supplementation. The current working model predicts that the majority of ADP transport is associated with creatine kinase activity (Aliev et al. 2011) and therefore in a small subset of animals (n = 5) we also assessed ADP transport in the presence of 20 mm creatine. While the apparent Km values were lower in the presence of creatine, they were not different in any group (LC, 102 ± 11; ZDF, 97 ± 14; Z-Resv, 135 ± 35). These data are consistent with the observation that permeabilized fibres from the atria of type 2 diabetic humans display normal ADP respiratory kinetics (Anderson et al. 2009a), further suggesting ADP transport is not affiliated with diabetic cardiomyopathy.
Figure 3. ADP respiratory kinetics in permeabilized muscle fibres from the left ventricle of LC, ZDF and Z-Resv rats.
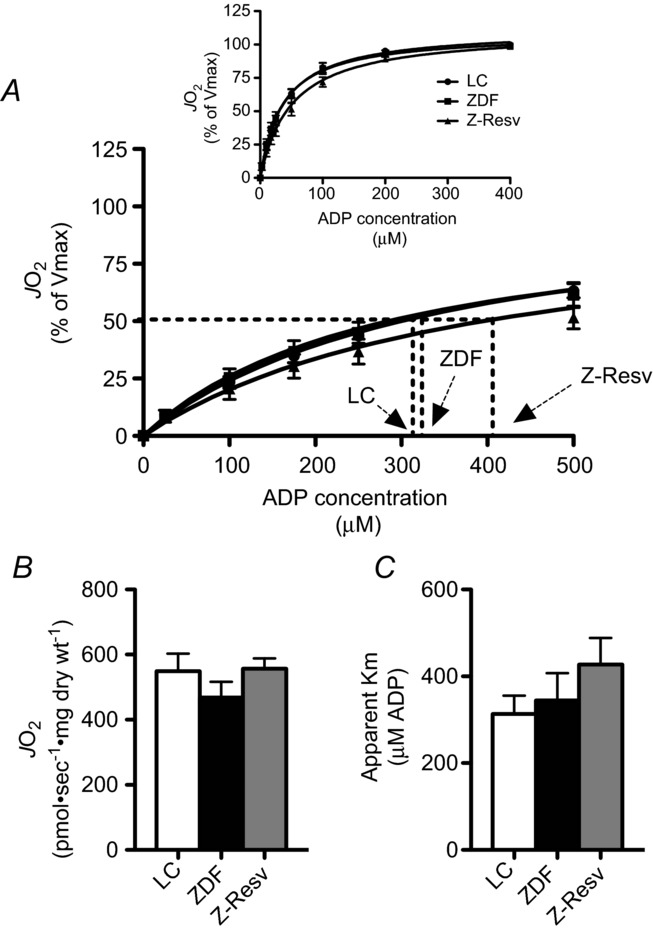
ADP titrated in the presence of 10 mm pyruvate and 2 mm malate revealed typical Michaelis–Menten kinetics (A). Maximal respiration (B; Vmax) and the apparent Km (C) were not different in any group. Values represent means ± SEM. n = 12–13 for determination of the apparent Km. A few fibres were not adequately recovered to determine maximal respiration normalized to dry weight, and therefore n = 8 for Vmax. JO2 = rate of oxygen consumption.
Complex I-supported substrate sensitivity in ZDF rats and following resveratrol supplementation
Given that ADP-respiratory sensitivity was not altered in the ZDF rat, we next determined the apparent respiratory sensitivity to pyruvate in the presence of 5 mm ADP. Similar to ADP titrations, substrate responses displayed typical Michaelis–Menten kinetics (Fig. 4A). Maximal pyruvate + malate-supported respiration was not different in any group studied (Fig. 4B), and was also similar to the Vmax reported for the reverse experiment (titrating ADP in presence of pyruvate + malate; Fig. 3B). In addition, the apparent sensitivity to pyruvate was not altered in any group (Fig. 4C). The unaltered maximal respiration following ADP and pyruvate titrations suggests the absence of mitochondrial biogenesis. This is supported by the finding that protein content was not altered in the subunits of the electron transport chain in any group (Fig. 5A–F). Altogether, these data strongly suggest an absence of changes within the electron transport chain in ZDF rats, or following resveratrol supplementation.
Figure 4. Pyruvate respiratory kinetics in permeabilized muscle fibres from the left ventricle of LC, ZDF and Z-Resv rats.
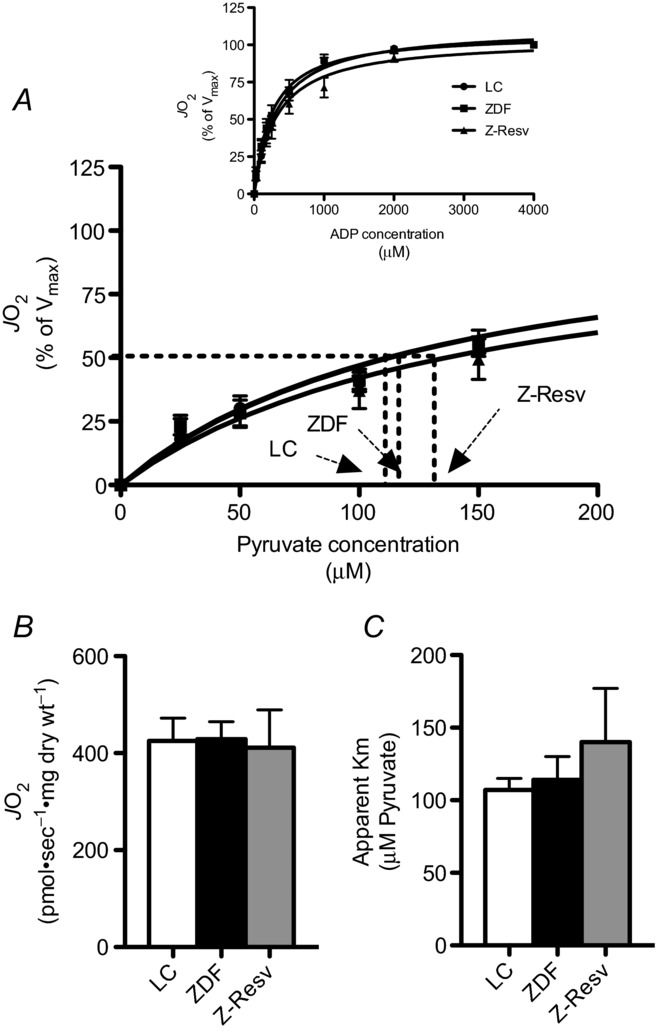
Pyruvate was titrated in the presence of 5 mm ADP and 2 mm malate revealed typical Michaelis–Menten kinetics (A). Maximal respiration (B; Vmax) and the apparent Km (C) were not different in any group. Values represent means ± SEM. n = 9–12 for determination of the apparent Km. A few fibres were not adequately recovered to determine maximal respiration normalized to dry weight, and therefore n = 6–7 for Vmax.
Figure 5. Markers of electron transport chain protein content in the left ventricle of LC, ZDF and Z-Resv rats.
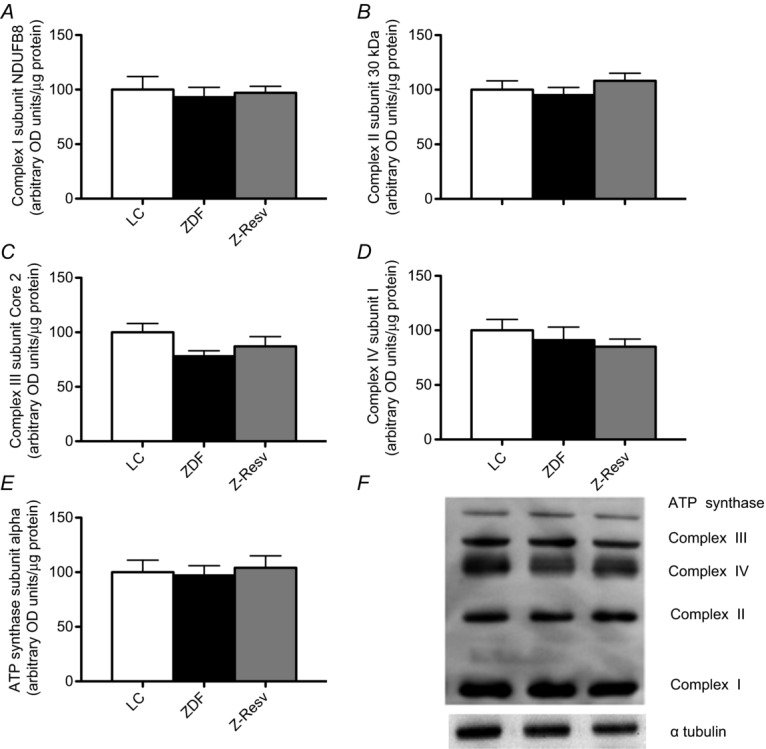
Complex I subunit NDUFB8 (A), complex II subunit 30 kDa (B), complex III subunit core 2 (C), complex IV subunit I (D), ATP synthase subunit α (E) were analysed and no significant differences were found. A representative OXPHOS blot, as well as α-tubulin which was used as a loading control, is shown in F. Values represent means ± SEM, n = 7 for all conditions, and 5 μg was loaded for all samples.
P-CoA sensitivity is impaired in ZDF rats and improved following resveratrol supplementation
The heart produces more than 90% of its energy aerobically, the majority of which is derived from lipid metabolism within mitochondria (Ventura-Clapier et al. 2011). We therefore next determined mitochondrial respiratory sensitivity to P-CoA, a naturally occurring lipid that represents the majority of lipid available for oxidation in vivo. While maximal P-CoA-supported respiration was not altered in ZDF rats (Fig. 6B), in contrast, the ZDF rats displayed an attenuation in P-CoA sensitivity (apparent Km increased ∼60%; Fig. 6C), supporting previous work showing a reduction in the expression of the liver isoform of carnitine palmitoyltransferase I (CPTI) at 7 weeks of age in ZDF rats (Zhou et al. 2000). Resveratrol supplementation increased maximal respiration ∼40% (Fig. 6B), while simultaneously recovering normal P-CoA respiratory sensitivity (Fig. 6C). Mitochondria in the hearts of ZDF rats therefore appear to have a specific dysfunction within either mitochondrial membrane lipid transport and/or β-oxidation that resveratrol fully recovers.
Figure 6. Palmitoyl-CoA (P-CoA) respiratory kinetics in permeabilized muscle fibres from the left ventricle of LC, ZDF and Z-Resv rats.
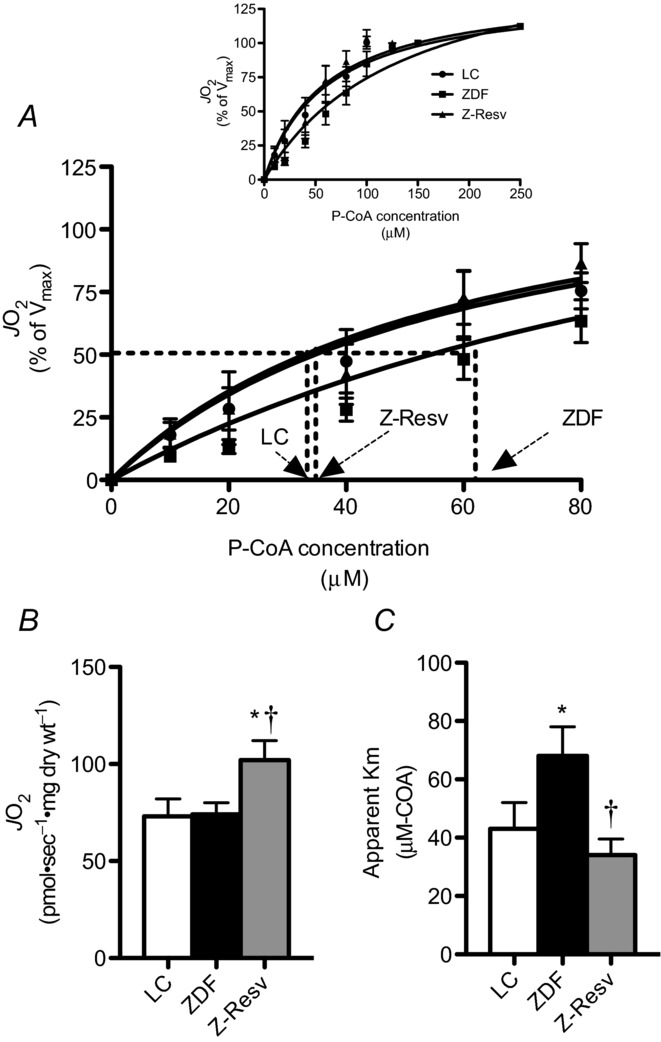
P-CoA titrated in the presence of 5 mm ADP, 2 mm malate and 2 mm l-carnitine revealed typical Michaelis–Menten kinetics (A). Maximal respiration (B; Vmax) and the apparent Km (C) were not different in any group. Values represent means ± SEM. n = 6–7 for determination of the apparent Km and Vmax. *Significantly (P < 0.05) different from LC. †Significantly (P < 0.05) different from ZDF.
Lipid accumulation is reduced in the hearts of ZDF rats following resveratrol supplementation
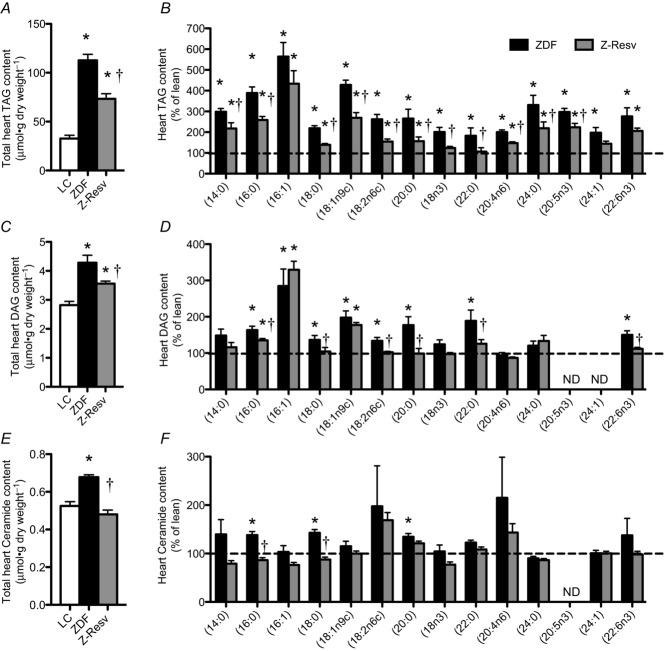
In healthy hearts, approximately 70–90% of the fatty acids taken up are immediately oxidized (Wisneski et al. 1987; Chandler et al. 2003), while only a small proportion is esterified to triacylglycerol (TAG). Nevertheless, an accumulation of TAG within the heart has been shown to occur in 6-week-old ZDF rats (Bonen et al. 2009) and is also associated with cardiac contractile dysfunction (Stanley et al. 2005). We therefore next aimed to determine if the observed improvements in mitochondrial P-CoA respiratory kinetics were associated with reductions in myocardial lipid species. Total heart TAG was increased ∼3.5-fold in the ZDF rat (Fig. 7A). While resveratrol decreased TAG content relative to untreated ZDF rats, it remained higher than lean control animals (Fig. 7A). The basic pattern of partial recovery of TAG following resveratrol supplementation was recapitulated in virtually all TAG species studied (Fig. 7B).
Figure 7. Left ventricular triacylglycerol (TAG), diacylglycerol (DAG) and ceramide contents in LC, ZDF and Z-Resv rats.
Total and various lipid species of TAG (A and B), DAG (C and D) and ceramides (E and F) were determined using gas chromatography. Values represent means ± SEM. n = 8 for all analyses. *Significantly (P < 0.05) different from LC. †Significantly (P < 0.05) different from ZDF.
Similar to the response for TAG, total heart diacylglycerol (DAG) was increased in the ZDF rat (Fig. 7A), while resveratrol partially recovered total DAG content (Fig. 7C). The basic pattern of partial recovery of DAG following resveratrol was present for 16:0, 18:0 and 18:2n6c DAG species, which represented ∼62% of the total DAG species detected (Fig. 7D). In contrast, 18:1n9c and 16:1, which represent ∼4% of total DAG, were not altered by resveratrol (Fig. 7D).
Similar to both TAG and DAG, ZDF rats displayed increased total ceramide content within cardiac muscle (Fig. 7E). However, in contrast to TAG and DAG responses, resveratrol fully normalized total ceramide content to lean control values (Fig. 7E). In addition, the uniform increase in almost all TAG and DAG species determined was not recapitulated in ceramide species as only 16:0, 18:0 and 20:0 ceramide species were increased in ZDF animals (Fig. 7F). Of these, 16:0 and 18:0 ceramide contents, which represent ∼60% of total ceramide detected, were normalized following resveratrol treatment (Fig. 7F). Altogether, in ZDF rats an impairement in lipid-supported respiratory kinetics is associated with lipid accumulation, while resveratrol normalizes both of these responses, suggesting a strong association between mitochondrial respiratory lipid kinetics and lipid accumulation within the heart.
Increased mitochondrial reactive oxygen species emission in the hearts of ZDF rats is recovered following resveratrol supplementation
Mitochondrial ROS emission is increased in permeabilized muscle fibres from diabetic db/db mice and in the atria of type 2 diabetic humans (Boudina et al. 2007; Anderson et al. 2009a), and is thought to contribute to the diabetic cardiomyopathy phenotype (Nishikawa et al. 2000). We therefore also examined maximal rates of succinate-induced ROS emission, and similar to these previous reports show that mitochondrial ROS emission was increased ∼3–fold in a model of diabetes (ZDF rat; Fig. 8A). We also show that resveratrol supplementation fully normalized mitochondrial ROS emission (Fig. 8A), supporting previous work showing resveratrol improved oxidative stress in diabetic models (Thirunavukkarasu et al. 2007; Qin et al. 2012; Soufi et al. 2012). While maximal electron transport chain ROS capacity was increased in the ZDF rat, this was not associated with alterations in the ratio of reduced/oxidized glutathione (total GSH and GSSG not altered; data not shown), a sensitive barometer of the in vivo redox state (Fig. 8B), or in the expression of SOD2 or CAT proteins (Fig. 8C and D, respectively). While redox stress is apparent in the heart of humans with type 2 diabetes (Anderson et al. 2009a), in db/db mice (Boudina et al. 2007), and in ZDF rats at 15 weeks of age (Guleria et al. 2013), the current data suggest that overt oxidative stress had not developed at this early stage in the ZDF disease progression.
Figure 8. Rates of mitochondrial hydrogen peroxide emission (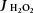 ), the ratio of reduced glutathione (GSH) to oxidized glutathione (GSSG) and antioxidant enzyme expression were determined in the left ventricle of LC, ZDF and Z-Resv rats.
), the ratio of reduced glutathione (GSH) to oxidized glutathione (GSSG) and antioxidant enzyme expression were determined in the left ventricle of LC, ZDF and Z-Resv rats.
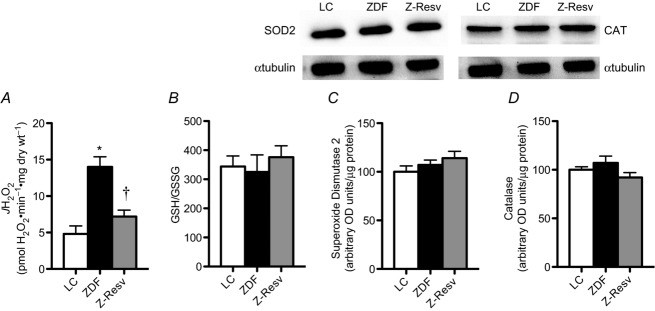
Rates of hydrogen peroxide were determine in permeabilized muscle fibres (A), while rapidly frozen whole muscle samples were used to determine GSH/GSSG (B) as well as the protein content of superoxide dismutase 2 (SOD2; C) and catalase (CAT; D) (OD, optical density). Values represent means ± SEM. n = 5–7. *Significantly (P < 0.05) different from LC. †Significantly (P < 0.05) different from ZDF.
Discussion
The current data provide evidence that before overt diabetic cardiomyopathy develops in ZDF rats there is (1) increased fibrosis, (2) decreased mitochondrial P-CoA sensitivity, (3) accumulation of reactive lipids, and (4) an increased propensity for mitochondrial ROS emission. Interestingly, the provision of resveratrol (5) completely normalizes almost all of these metabolic derangements and therefore provides further understanding to the molecular benefits of resveratrol in improving cardiovascular health in the context of diabetes.
Fibrosis has a well-defined relationship with changes in rates of relaxation and compromised ventricular function. In contrast, the biological significance of impaired P-CoA sensitivity is currently unknown. Chronically, attenuated P-CoA sensitivity may result in lipid accumulation within the heart and diastolic dysfunction, and therefore be maladaptive and contribute to the development of diabetic cardiomyopathy. This is supported by the finding that heterozygous CPTIb mice challenged with a pressure overload display lipotoxicity, impaired stroke volume and cardiac output, and increased fibrosis (He et al. 2012). However acutely, an attenuation in P-CoA sensitivity may be adaptive to prevent excessive fatty acid accumulation within mitochondria and the subsequent generation of ROS. This interpretation may help explain why pharmacological approaches that decrease lipid transport into mitochondria are beneficial in the context of diabetic cardiomyopathy (Schmitz et al. 1995; Turcani & Rupp, 1997; Lee et al. 2005; Abozguia et al. 2010).
While convenient to conclude that attenuated P-CoA respiratory kinetics would directly impair contractile function, ZDF hearts display an increased rate of fatty acid oxidation (Wang et al. 2005; van den Brom et al. 2009). However, in various models, including ZDF rats, rates of plasma membrane long-chain fatty acid transport are increased, and therefore lipid ‘delivery’ to mitochondria enhanced (Bonen et al. 2009). The transport of lipids across the plasma membrane is now recognized to be a protein-mediated process that is highly regulated, and the increase in lipid transport into the heart of ZDF rats appears to involve the translocation of fatty acid translocase (FAT/CD36) to the plasma membrane (Bonen et al. 2009). This molecular event occurs early in the disease progression, as the ZDF rat displays an increase in plasma membrane FAT/CD36 at 6 weeks of age (Bonen et al. 2009). Therefore, the attenuation in mitochondrial P-CoA sensitivity observed in the current study may not affect contractile function or impair rates of fatty acid oxidation at the whole heart level, and instead maintain the appropriate absolute quantity of lipid transported into mitochondria. In a high-fat environment mitochondrially derived ROS are primarily thought to result from lipid oxidation, as mitochondrial ROS emission occurs at a very low lipid concentration, an observation attributed to the fact that the electron transfer flavoprotein (ETF) involved in β-oxidation is membrane potential independent (Seifert et al. 2010). However, in a model known to have increased lipid delivery into cardiac muscle (Bonen et al. 2009), in the current study, despite an increase in the capacity of the electron transport chain for ROS emission and unaltered SOD2 and CAT protein content, a sensitive barometer of the in vivo redox state (GSH/GSSG) was not altered in the ZDF rat, suggesting the absence of overt oxidative stress. Altogether, these data suggest that limiting fatty acid transport into mitochondria may be beneficial in the context of maintaining redox balance. Interestingly, resveratrol, a polyphenol compound that has inherent antioxidant properties, decreased the capacity for mitochondrial ROS emission as well as normalized P-CoA respiratory sensitivity. It is possible that ROS mediates the observed attenuation in P-CoA sensitivity as a feedback mechanism to prevent excessive lipid accumulation within mitochondria and overt oxidative damage. Therefore, attenuating lipid transport into mitochondria may acutely maintain redox balance, and cardiac function. However, the obvious implications chronically to a reduction in P-CoA sensitivity involve impaired aerobic lipid metabolism, reactive lipid accumulation and compromised ventricular function.
Impaired ADP respiratory sensitivity is a common observation in various models of pressure-induced heart failure (Ventura-Clapier et al. 2011); however, this was not observed in the current model of early-stage diabetic cardiomyopathy. The attenuation in the apparent ADP respiratory Km in other heart failure models appears to primarily occur as a result of a reduction in the expression of mitochondrial creatine kinase and adenine nucleotide translocase (ANT) (Ventura-Clapier et al. 2011). However, P-CoA has also been shown to interact with ANT, a required inner mitochondrial membrane ADP transporter. Specifically, long-chain acyl-CoA moieties have been shown to directly suppress ADP transport by competitively binding to the ADP binding site on ANT (Ho & Pande, 1974; Woldegiorgis & Shrago, 1979). Therefore, even in an absence of altered gene transcription of mitochondrial creatine kinase or ANT, a chronic attenuation in P-CoA respiratory sensitivity would be expected to impair ADP transport as a result of increased cytosolic and/or matrix P-CoA concentrations. This would be expected to further compromise aerobic respiration within the heart, and exaggerate an existing metabolic dysfunction. However, to date ‘lipid’- and ‘carbohydrate’-supported mitochondrial bioenergetics have been exclusively assessed independently, the current study included. Therefore, future work should consider assessing mitochondrial ADP-respiratory sensitivity in the presence of small quantities of P-CoA to better reflect the in vivo situation in hearts from diabetic animals.
Resveratrol, a polyphenol compound, has been shown to exert pleiotropic actions in several tissues that may be beneficial in the context of diabetic cardiomyopathy. Specifically, resveratrol has been shown to improve whole body glucose tolerance (Beaudoin et al. 2013), skeletal muscle glucose uptake (Smith et al. 2013), white adipose tissue metabolism (Beaudoin et al. 2013), and several derangements associated with hypertension (Rimbaud et al. 2011), myocardial infarction (Chen et al. 2008), ischaemia/reperfusion (Lin et al. 2008) and high-fat-diet-induced diabetic cardiomyopathy (Louis et al. 2012; Qin et al. 2012). In the context of diet-induced cardiac pathologies, resveratrol has been shown to prevent fibrosis, cellular oxidative stress and diastolic dysfunction (Louis et al. 2012; Qin et al. 2012). We extend these findings by suggesting resveratrol partially mediates these effects by improving mitochondrial bioenergetics. Specifically, we show that resveratrol normalized P-CoA respiratory sensitivity, increased maximal rates of P-CoA respiration and decreased maximal succinate-induced mitochondrial ROS emission. In addition, the previous observations that resveratrol improved cardiac redox balance were somewhat ambiguous (Louis et al. 2012; Qin et al. 2012), as it was not clear if this protection manifested from changes within cardiac tissue, or simply a response to systemic adaptations. However, the current study clearly shows that the increased propensity for mitochondrial ROS emission in ZDF rats is recovered following resveratrol supplementation, suggesting intrinsic changes within the heart contributes to the previously observed resveratrol-induced improvement in redox balance, although systemic adaptions are probably involved as well. The reduction in mitochondrial H2O2 emission in the current study, in combination with the antioxidant properties of resveratrol, probably contribute to the improvement in cardiac fibrosis, as decreasing cellular ROS production prevents cardiac fibrosis (Liu et al. 2010), while decreasing the antioxidant capacity of the heart (SOD1-deficient mice) induces gene expression patterns associated with fibrosis (Hagler et al. 2013).
In addition, in the current study, the recovery of mitochondrial P-CoA respiratory sensitivity may contribute to the normalization of reactive lipids in ZDF animals. Importantly, the cytotoxic effects of ceramides are well characterized in cardiomyocytes (Park et al. 2008), as ceramide induces apoptosis (Okere et al. 2006), probably aggravating the pathological development of diabetic cardiomyopathy, which is known to manifest with a decrease in mitochondrial content chronically (Ventura-Clapier et al. 2011). In the current study, ZDF rats primarily displayed an increase in 16:0 and 18:0 derived ceramides, which have also been reported to be elevated in CPTI heterozygous-induced cardiac pathologies (He et al. 2012). Therefore, there appears to be a clear association between impaired lipid transport into mitochondria and the accumulation of specific ceramide species. In combination with normalizing P-CoA respiratory kinetics, resveratrol fully normalized total ceramide content as well as 16:0 and 18:0 derived ceramide species. Therefore, resveratrol improved all aspects of mitochondrial bioenergetics and reactive lipid profiles in hearts from the ZDF rat.
Altogether the current data support the supposition that both cardiac fibrosis and a chronic dysfunction within mitochondrial lipid-supported bioenergetics contribute to the development of diabetic cardiomyopathy. Specifically, we show that P-CoA respiratory sensitivity is impaired in ZDF rats, which coincides with an accumulation of intramyocellular lipids and cardiac fibrosis. In addition, we show resveratrol supplementation prevents these changes, supporting the belief that resveratrol is a potent therapeutic approach for preventing diabetic cardiomyopathy.
Acknowledgments
None.
Glossary
- ANT
adenine nucleotide translocase
- CAT
catalase
- CPTI
carnitine palmitoyltransferase I
- DAG
diacylglycerol
- +dP/dt
maximal rates of pressure development
- –dP/dt
maximal rates of pressure decline
- EDP
end-diastolic pressure
- GSH
reduced glutathione
- GSSG
oxidized glutathione
- LC
lean control
- LVP max
maximal left ventricular pressure
- P-CoA
palmitoyl-CoA
- ROS
reactive oxygen species
- SOD2
superoxide dismutase 2
- TAG
triacylglycerol
- ZDF rat
Zucker diabetic fatty rat
- Z-Resv rat
Zucker diabetic fatty rat supplemented with resveratrol
Additional information
Competing interests
The authors have no conflict of interest to disclose.
Author contributions
G.P.H. designed experiments, performed experiments, analysed and interpreted data and wrote the manuscript. M.-S.B., C.G.R.P., D.C.W., J.A.S., A.A. and A.C. performed experiments, analysed data and edited the manuscript. All authors have read and approved the final version of the manuscript. All experiments were conducted at the University of Guelph, with the exception of the GC lipid analyses which were conducted at the Medical University of Bialystok.
Funding
This work was funded by The Natural Sciences and Engineering Research Council of Canada (NSERC; G.P.H., D.C.W. and J.A.S.) and CIHR (D.C.W. and J.A.S.) and equipment was purchased with the assistance of the Canadian Foundation for Innovation (G.P.H., D.C.W. and J.A.S.) as well as the Ontario Research Fund (G.P.H., D.C.W. and J.A.S.). D.C.W. is a Canada Research Chair.
References
- Abozguia K, Elliott P, McKenna W, Phan TT, Nallur-Shivu G, Ahmed I, Maher AR, Kaur K, Taylor J, Henning A, Ashrafian H, Watkins H, Frenneaux M. Metabolic modulator perhexiline corrects energy deficiency and improves exercise capacity in symptomatic hypertrophic cardiomyopathy. Circulation. 2010;122:1562–1569. doi: 10.1161/CIRCULATIONAHA.109.934059. [DOI] [PubMed] [Google Scholar]
- Aliev M, Guzun R, Karu-Varikmaa M, Kaambre T, Wallimann T, Saks V. Molecular system bioenergics of the heart: experimental studies of metabolic compartmentation and energy fluxes versus computer modeling. Int J Mol Sci. 2011;12:9296–9331. doi: 10.3390/ijms12129296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson EJ, Kypson AP, Rodriguez E, Anderson CA, Lehr EJ, Neufer PD. Substrate-specific derangements in mitochondrial metabolism and redox balance in the atrium of the type 2 diabetic human heart. J Am Coll Cardiol. 2009a;54:1891–1898. doi: 10.1016/j.jacc.2009.07.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson EJ, Lustig ME, Boyle KE, Woodlief TL, Kane DA, Lin CT, Price JW, 3rd, Kang L, Rabinovitch PS, Szeto HH, Houmard JA, Cortright RN, Wasserman DH, Neufer PD. Mitochondrial H2O2 emission and cellular redox state link excess fat intake to insulin resistance in both rodents and humans. J Clin Invest. 2009b;119:573–581. doi: 10.1172/JCI37048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Augustus AS, Buchanan J, Park TS, Hirata K, Noh HL, Sun J, Homma S, D'Armiento J, Abel ED, Goldberg IJ. Loss of lipoprotein lipase-derived fatty acids leads to increased cardiac glucose metabolism and heart dysfunction. J Biol Chem. 2006;281:8716–8723. doi: 10.1074/jbc.M509890200. [DOI] [PubMed] [Google Scholar]
- Baynes J, Murray DB. Cardiac and renal function are progressively impaired with aging in Zucker diabetic fatty type II diabetic rats. Oxid Med Cell Longev. 2009;2:328–334. doi: 10.4161/oxim.2.5.9831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beaudoin MS, Snook LA, Arkell AM, Simpson JA, Holloway GP, Wright DC. Resveratrol supplementation improves white adipose tissue function in a depot-specific manner in Zucker diabetic fatty rats. Am J Physiol Regul Integr Comp Physiol. 2013;305:R542–R551. doi: 10.1152/ajpregu.00200.2013. [DOI] [PubMed] [Google Scholar]
- Belke DD, Larsen TS, Gibbs EM, Severson DL. Altered metabolism causes cardiac dysfunction in perfused hearts from diabetic (dbdb) mice. Am J Physiol Endocrinol Metab. 2000;279:E1104–E1113. doi: 10.1152/ajpendo.2000.279.5.E1104. [DOI] [PubMed] [Google Scholar]
- Bonen A, Holloway GP, Tandon NN, Han XX, McFarlan J, Glatz JF, Luiken JJ. Cardiac and skeletal muscle fatty acid transport and transporters and triacylglycerol and fatty acid oxidation in lean and Zucker diabetic fatty rats. Am J Physiol Regul Integr Comp Physiol. 2009;297:R1202–R1212. doi: 10.1152/ajpregu.90820.2008. [DOI] [PubMed] [Google Scholar]
- Boudina S, Sena S, Theobald H, Sheng X, Wright JJ, Hu XX, Aziz S, Johnson JI, Bugger H, Zaha VG, Abel ED. Mitochondrial energetics in the heart in obesity-related diabetes: direct evidence for increased uncoupled respiration and activation of uncoupling proteins. Diabetes. 2007;56:2457–2466. doi: 10.2337/db07-0481. [DOI] [PubMed] [Google Scholar]
- Bugger H, Chen D, Riehle C, Soto J, Theobald HA, Hu XX, Ganesan B, Weimer BC, Abel ED. Tissue-specific remodeling of the mitochondrial proteome in type 1 diabetic Akita mice. Diabetes. 2009;58:1986–1997. doi: 10.2337/db09-0259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chandler MP, Chavez PN, McElfresh TA, Huang H, Harmon CS, Stanley WC. Partial inhibition of fatty acid oxidation increases regional contractile power and efficiency during demand-induced ischemia. Cardiovasc Res. 2003;59:143–151. doi: 10.1016/s0008-6363(03)00327-4. [DOI] [PubMed] [Google Scholar]
- Chen YR, Yi FF, Li XY, Wang CY, Chen L, Yang XC, Su PX, Cai J. Resveratrol attenuates ventricular arrhythmias and improves the long-term survival in rats with myocardial infarction. Cardiovasc Drugs Ther. 2008;22:479–485. doi: 10.1007/s10557-008-6141-8. [DOI] [PubMed] [Google Scholar]
- Coort SL, Hasselbaink DM, Koonen DP, Willems J, Coumans WA, Chabowski A, van der Vusse GJ, Bonen A, Glatz JF, Luiken JJ. Enhanced sarcolemmal FAT/CD36 content and triacylglycerol storage in cardiac myocytes from obese Zucker rats. Diabetes. 2004;53:1655–1663. doi: 10.2337/diabetes.53.7.1655. [DOI] [PubMed] [Google Scholar]
- Daniels A, Linz D, van Bilsen M, Rutten H, Sadowski T, Ruf S, Juretschke HP, Neumann-Haefelin C, Munts C, van der Vusse GJ, van Nieuwenhoven FA. Long-term severe diabetes only leads to mild cardiac diastolic dysfunction in Zucker diabetic fatty rats. Eur J Heart Fail. 2012;14:193–201. doi: 10.1093/eurjhf/hfr166. [DOI] [PubMed] [Google Scholar]
- Daniels A, van Bilsen M, Janssen BJ, Brouns AE, Cleutjens JP, Roemen TH, Schaart G, van der Velden J, van der Vusse GJ, van Nieuwenhoven FA. Impaired cardiac functional reserve in type 2 diabetic dbdb mice is associated with metabolic, but not structural, remodelling. Acta Physiol (Oxf) 2010;200:11–22. doi: 10.1111/j.1748-1716.2010.02102.x. [DOI] [PubMed] [Google Scholar]
- Delucchi F, Berni R, Frati C, Cavalli S, Graiani G, Sala R, Chaponnier C, Gabbiani G, Calani L, Del Rio D, Bocchi L, Lagrasta C, Quaini F, Stilli D. Resveratrol treatment reduces cardiac progenitor cell dysfunction and prevents morpho-functional ventricular remodeling in type–1 diabetic rats. PLoS One. 2012;7:e39836. doi: 10.1371/journal.pone.0039836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finck BN, Han X, Courtois M, Aimond F, Nerbonne JM, Kovacs A, Gross RW, Kelly DP. A critical role for PPARα-mediated lipotoxicity in the pathogenesis of diabetic cardiomyopathy: modulation by dietary fat content. Proc Natl Acad Sci U S A. 2003;100:1226–1231. doi: 10.1073/pnas.0336724100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fisher-Wellman KH, Mattox TA, Thayne K, Katunga LA, La Favor JD, Neufer PD, Hickner RC, Wingard CJ, Anderson EJ. Novel role for thioredoxin reductase–2 in mitochondrial redox adaptations to obesogenic diet and exercise in heart and skeletal muscle. J Physiol. 2013;591:3471–3486. doi: 10.1113/jphysiol.2013.254193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guleria RS, Singh AB, Nizamutdinova IT, Souslova T, Mohammad AA, Kendall JA, Jr, Baker KM, Pan J. Activation of retinoid receptor-mediated signalling ameliorates diabetes-induced cardiac dysfunction in Zucker diabetic rats. J Mol Cell Cardiol. 2013;57:106–118. doi: 10.1016/j.yjmcc.2013.01.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hagler MA, Hadley TM, Zhang H, Mehra K, Roos CM, Schaff HV, Suri RM, Miller JD. TGF-β signalling and reactive oxygen species drive fibrosis and matrix remodelling in myxomatous mitral valves. Cardiovasc Res. 2013;99:175–184. doi: 10.1093/cvr/cvt083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He L, Kim T, Long Q, Liu J, Wang P, Zhou Y, Ding Y, Prasain J, Wood PA, Yang Q. Carnitine palmitoyltransferase-1b deficiency aggravates pressure overload-induced cardiac hypertrophy caused by lipotoxicity. Circulation. 2012;126:1705–1716. doi: 10.1161/CIRCULATIONAHA.111.075978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ho CH, Pande SV. On the specificity of the inhibition of adenine nucleotide translocase by long chain acyl-coenzyme A esters. Biochim Biophys Acta. 1974;369:86–94. doi: 10.1016/0005-2760(74)90195-7. [DOI] [PubMed] [Google Scholar]
- Lagouge M, Argmann C, Gerhart-Hines Z, Meziane H, Lerin C, Daussin F, Messadeq N, Milne J, Lambert P, Elliott P, Geny B, Laakso M, Puigserver P, Auwerx J. Resveratrol improves mitochondrial function and protects against metabolic disease by activating SIRT1 and PGC-1α. Cell. 2006;127:1109–1122. doi: 10.1016/j.cell.2006.11.013. [DOI] [PubMed] [Google Scholar]
- Lee L, Campbell R, Scheuermann-Freestone M, Taylor R, Gunaruwan P, Williams L, Ashrafian H, Horowitz J, Fraser AG, Clarke K, Frenneaux M. Metabolic modulation with perhexiline in chronic heart failure: a randomized, controlled trial of short-term use of a novel treatment. Circulation. 2005;112:3280–3288. doi: 10.1161/CIRCULATIONAHA.105.551457. [DOI] [PubMed] [Google Scholar]
- Lin JF, Lin SM, Chih CL, Nien MW, Su HH, Hu BR, Huang SS, Tsai SK. Resveratrol reduces infarct size and improves ventricular function after myocardial ischemia in rats. Life Sci. 2008;83:313–317. doi: 10.1016/j.lfs.2008.06.016. [DOI] [PubMed] [Google Scholar]
- Liu Y, Huang H, Xia W, Tang Y, Li H, Huang C. NADPH oxidase inhibition ameliorates cardiac dysfunction in rabbits with heart failure. Mol Cell Biochem. 2010;343:143–153. doi: 10.1007/s11010-010-0508-4. [DOI] [PubMed] [Google Scholar]
- Louis XL, Thandapilly SJ, MohanKumar SK, Yu L, Taylor CG, Zahradka P, Netticadan T. Treatment with low-dose resveratrol reverses cardiac impairment in obese prone but not in obese resistant rats. J Nutr Biochem. 2012;23:1163–1169. doi: 10.1016/j.jnutbio.2011.06.010. [DOI] [PubMed] [Google Scholar]
- Marsh SA, Powell PC, Agarwal A, Dell’Italia LJ, Chatham JC. Cardiovascular dysfunction in Zucker obese and Zucker diabetic fatty rats: role of hydronephrosis. Am J Physiol Heart Circ Physiol. 2007;293:H292–H298. doi: 10.1152/ajpheart.01362.2006. [DOI] [PubMed] [Google Scholar]
- Mazumder PK, O'Neill BT, Roberts MW, Buchanan J, Yun UJ, Cooksey RC, Boudina S, Abel ED. Impaired cardiac efficiency and increased fatty acid oxidation in insulin-resistant obob mouse hearts. Diabetes. 2004;53:2366–2374. doi: 10.2337/diabetes.53.9.2366. [DOI] [PubMed] [Google Scholar]
- Neitzel AS, Carley AN, Severson DL. Chylomicron and palmitate metabolism by perfused hearts from diabetic mice. Am J Physiol Endocrinol Metab. 2003;284:E357–E365. doi: 10.1152/ajpendo.00380.2002. [DOI] [PubMed] [Google Scholar]
- Nishikawa T, Edelstein D, Du XL, Yamagishi S, Matsumura T, Kaneda Y, Yorek MA, Beebe D, Oates PJ, Hammes HP, Giardino I, Brownlee M. Normalizing mitochondrial superoxide production blocks three pathways of hyperglycaemic damage. Nature. 2000;404:787–790. doi: 10.1038/35008121. [DOI] [PubMed] [Google Scholar]
- Okere IC, Chandler MP, McElfresh TA, Rennison JH, Sharov V, Sabbah HN, Tserng KY, Hoit BD, Ernsberger P, Young ME, Stanley WC. Differential effects of saturated and unsaturated fatty acid diets on cardiomyocyte apoptosis, adipose distribution, and serum leptin. Am J Physiol Heart Circ Physiol. 2006;291:H38–H44. doi: 10.1152/ajpheart.01295.2005. [DOI] [PubMed] [Google Scholar]
- Park TS, Hu Y, Noh HL, Drosatos K, Okajima K, Buchanan J, Tuinei J, Homma S, Jiang XC, Abel ED, Goldberg IJ. Ceramide is a cardiotoxin in lipotoxic cardiomyopathy. J Lipid Res. 2008;49:2101–2112. doi: 10.1194/jlr.M800147-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perry CG, Kane DA, Herbst EA, Mukai K, Lark DS, Wright DC, Heigenhauser GJ, Neufer PD, Spriet LL, Holloway GP. Mitochondrial creatine kinase activity and phosphate shuttling are acutely regulated by exercise in human skeletal muscle. J Physiol. 2012;590:5475–5486. doi: 10.1113/jphysiol.2012.234682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perry CG, Kane DA, Lin CT, Kozy R, Cathey BL, Lark DS, Kane CL, Brophy PM, Gavin TP, Anderson EJ, Neufer PD. Inhibiting myosin-ATPase reveals a dynamic range of mitochondrial respiratory control in skeletal muscle. Biochem J. 2011;437:215–222. doi: 10.1042/BJ20110366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qin F, Siwik DA, Luptak I, Hou X, Wang L, Higuchi A, Weisbrod RM, Ouchi N, Tu VH, Calamaras TD, Miller EJ, Verbeuren TJ, Walsh K, Cohen RA, Colucci WS. The polyphenols resveratrol and S17834 prevent the structural and functional sequelae of diet-induced metabolic heart disease in mice. Circulation. 2012;125:1757–1764. doi: 10.1161/CIRCULATIONAHA.111.067801. S1751–1756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Radovits T, Korkmaz S, Loganathan S, Barnucz E, Bomicke T, Arif R, Karck M, Szabo G. Comparative investigation of the left ventricular pressure-volume relationship in rat models of type 1 and type 2 diabetes mellitus. Am J Physiol Heart Circ Physiol. 2009;297:H125–H133. doi: 10.1152/ajpheart.00165.2009. [DOI] [PubMed] [Google Scholar]
- Rimbaud S, Ruiz M, Piquereau J, Mateo P, Fortin D, Veksler V, Garnier A, Ventura-Clapier R. Resveratrol improves survival, hemodynamics and energetics in a rat model of hypertension leading to heart failure. PLoS One. 2011;6:e26391. doi: 10.1371/journal.pone.0026391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robich MP, Chu LM, Burgess TA, Feng J, Han Y, Nezafat R, Leber MP, Laham RJ, Manning WJ, Sellke FW. Resveratrol preserves myocardial function and perfusion in remote nonischemic myocardium in a swine model of metabolic syndrome. J Am Coll Surg. 2012;215:681–689. doi: 10.1016/j.jamcollsurg.2012.06.417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schäfer S, Huber J, Wihler C, Rutten H, Busch AE, Linz W. Impaired left ventricular relaxation in type 2 diabetic rats is related to myocardial accumulation of Nε-(carboxymethyl) lysine. Eur J Heart Fail. 2006;8:2–6. doi: 10.1016/j.ejheart.2005.04.011. [DOI] [PubMed] [Google Scholar]
- Schmitz FJ, Rosen P, Reinauer H. Improvement of myocardial function and metabolism in diabetic rats by the carnitine palmitoyl transferase inhibitor Etomoxir. Horm Metab Res. 1995;27:515–522. doi: 10.1055/s-2007-980016. [DOI] [PubMed] [Google Scholar]
- Seifert EL, Estey C, Xuan JY, Harper ME. Electron transport chain-dependent and -independent mechanisms of mitochondrial H2O2 emission during long-chain fatty acid oxidation. J Biol Chem. 2010;285:5748–5758. doi: 10.1074/jbc.M109.026203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith BK, Perry CG, Herbst EA, Ritchie IR, Beaudoin MS, Smith JC, Neufer PD, Wright DC, Holloway GP. Submaximal ADP-stimulated respiration is impaired in ZDF rats and recovered by resveratrol. J Physiol. 2013;591:6089–6101. doi: 10.1113/jphysiol.2013.259226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith BK, Perry CG, Koves TR, Wright DC, Smith JC, Neufer PD, Muoio DM, Holloway GP. Identification of a novel malonyl-CoA IC50 for CPT–I: implications for predicting in vivo fatty acid oxidation rates. Biochem J. 2012;448:13–20. doi: 10.1042/BJ20121110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soufi FG, Vardyani M, Sheervalilou R, Mohammadi M, Somi MH. Long-term treatment with resveratrol attenuates oxidative stress pro-inflammatory mediators and apoptosis in streptozotocin-nicotinamide-induced diabetic rats. Gen Physiol Biophys. 2012;31:431–438. doi: 10.4149/gpb_2012_039. [DOI] [PubMed] [Google Scholar]
- Stanley WC, Lopaschuk GD, McCormack JG. Regulation of energy substrate metabolism in the diabetic heart. Cardiovasc Res. 1997;34:25–33. doi: 10.1016/s0008-6363(97)00047-3. [DOI] [PubMed] [Google Scholar]
- Stanley WC, Recchia FA, Lopaschuk GD. Myocardial substrate metabolism in the normal and failing heart. Physiol Rev. 2005;85:1093–1129. doi: 10.1152/physrev.00006.2004. [DOI] [PubMed] [Google Scholar]
- Thirunavukkarasu M, Penumathsa SV, Koneru S, Juhasz B, Zhan L, Otani H, Bagchi D, Das DK, Maulik N. Resveratrol alleviates cardiac dysfunction in streptozotocin-induced diabetes: Role of nitric oxide, thioredoxin, and heme oxygenase. Free Radic Biol Med. 2007;43:720–729. doi: 10.1016/j.freeradbiomed.2007.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turcani M, Rupp H. Etomoxir improves left ventricular performance of pressure-overloaded rat heart. Circulation. 1997;96:3681–3686. doi: 10.1161/01.cir.96.10.3681. [DOI] [PubMed] [Google Scholar]
- Tuunanen H, Engblom E, Naum A, Nagren K, Hesse B, Airaksinen KE, Nuutila P, Iozzo P, Ukkonen H, Opie LH, Knuuti J. Free fatty acid depletion acutely decreases cardiac work and efficiency in cardiomyopathic heart failure. Circulation. 2006;114:2130–2137. doi: 10.1161/CIRCULATIONAHA.106.645184. [DOI] [PubMed] [Google Scholar]
- van den Brom CE, Huisman MC, Vlasblom R, Boontje NM, Duijst S, Lubberink M, Molthoff CF, Lammertsma AA, van der Velden J, Boer C, Ouwens DM, Diamant M. Altered myocardial substrate metabolism is associated with myocardial dysfunction in early diabetic cardiomyopathy in rats: studies using positron emission tomography. Cardiovasc Diabetol. 2009;8:39. doi: 10.1186/1475-2840-8-39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ventura-Clapier R, Garnier A, Veksler V, Joubert F. Bioenergetics of the failing heart. Biochim Biophys Acta. 2011;1813:1360–1372. doi: 10.1016/j.bbamcr.2010.09.006. [DOI] [PubMed] [Google Scholar]
- Wang P, Lloyd SG, Zeng H, Bonen A, Chatham JC. Impact of altered substrate utilization on cardiac function in isolated hearts from Zucker diabetic fatty rats. Am J Physiol Heart Circ Physiol. 2005;288:H2102–H2110. doi: 10.1152/ajpheart.00935.2004. [DOI] [PubMed] [Google Scholar]
- Wisneski JA, Gertz EW, Neese RA, Mayr M. Myocardial metabolism of free fatty acids. Studies with 14C-labelled substrates in humans. J Clin Invest. 1987;79:359–366. doi: 10.1172/JCI112820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woldegiorgis G, Shrago E. The recognition of two specific binding sites of the adenine nucleotide translocase by palmitoyl CoA in bovine heart mitochondria and submitochondrial particles. Biochem Biophys Res Commun. 1979;89:837–844. doi: 10.1016/0006-291x(79)91854-0. [DOI] [PubMed] [Google Scholar]
- Yagyu H, Chen G, Yokoyama M, Hirata K, Augustus A, Kako Y, Seo T, Hu Y, Lutz EP, Merkel M, Bensadoun A, Homma S, Goldberg IJ. Lipoprotein lipase (LpL) on the surface of cardiomyocytes increases lipid uptake and produces a cardiomyopathy. J Clin Invest. 2003;111:419–426. doi: 10.1172/JCI16751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou YT, Grayburn P, Karim A, Shimabukuro M, Higa M, Baetens D, Orci L, Unger RH. Lipotoxic heart disease in obese rats: implications for human obesity. Proc Natl Acad Sci U S A. 2000;97:1784–1789. doi: 10.1073/pnas.97.4.1784. [DOI] [PMC free article] [PubMed] [Google Scholar]