Figure 3.
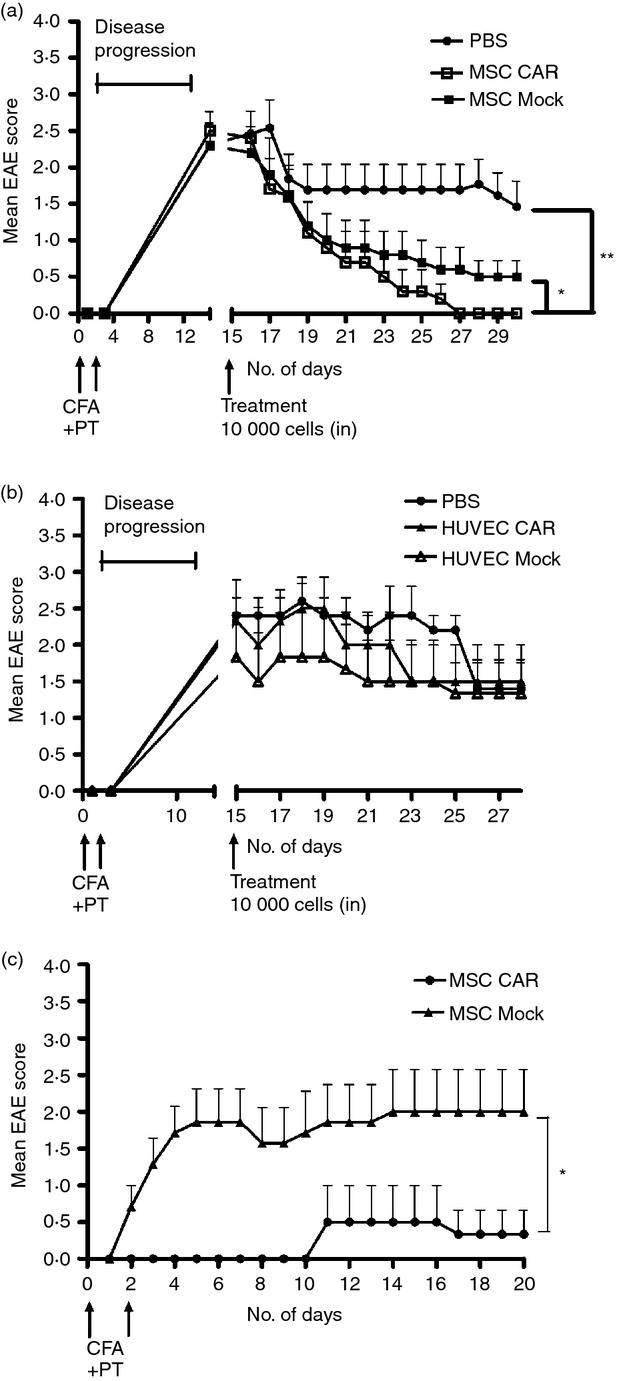
Efficacy of central nervous system (CNS) -targeted mesenchymal stromal cells (MSC)s in experimental autoimmune encephalomyelitis (EAE) mice. (a) Ten EAE mice in three groups were given a low dose (1 × 104) of engineered CARαMOG or Mock MSCs or PBS alone by intranasal (i.n.) administration at the peak of EAE inflammation (15 days post-EAE immunization). Ten days after i.n. MSC treatment all mice in the CARαMOG group were symptom-free (**P < 0·01). At end-point (15 days after i.n. MSC treatment) mice in the mock-treated group still exhibited EAE symptoms (P < 0·05). The experiment was repeated three times with similar results. (b) To exclude that treatment efficacy was due to a xenogeneic response of the human MSC, mice were treated with human HUVEC cells equipped with the CARαMOG targeting receptor. Six mice in three groups were given a low dose (1 × 104) of engineered CARαMOG or Mock HUVECs, or PBS alone by i.n. delivery at the peak of EAE inflammation (15 days post-EAE immunization). (c) Cured EAE mice from each treatment group of i.n. MSCs (n = 6) were given a second EAE immunization (as described previously). MSC CARαMOG-treated mice were able to resist EAE inflammation longer than MSC-mock-treated mice (*P < 0·05). Statistics were analysed using the Mann–Whitney U-test and GraphPad prism software.
