Figure 5.
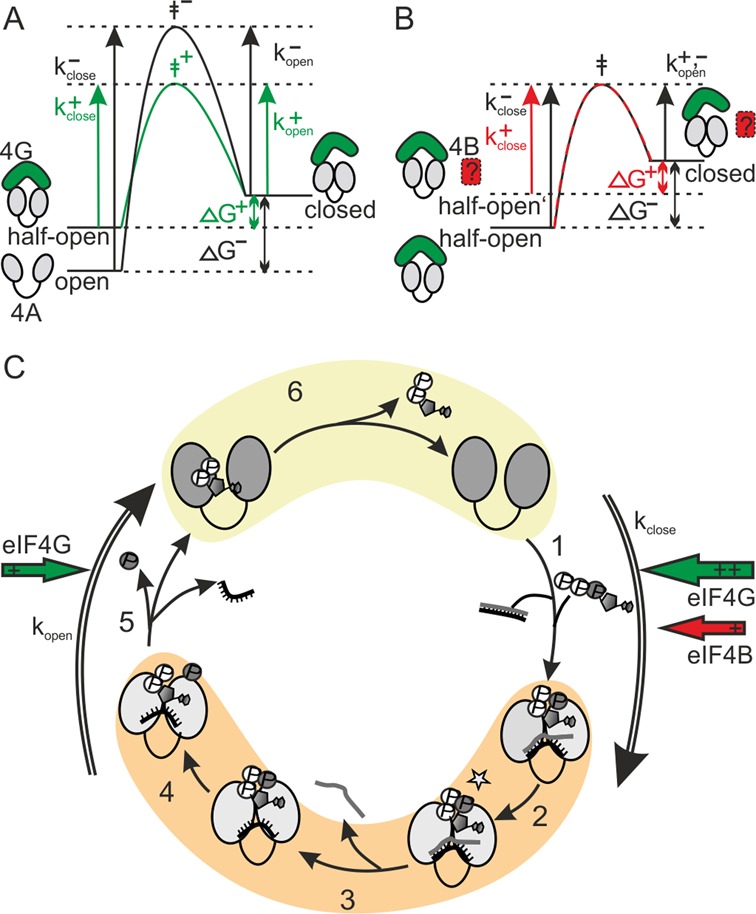
Model for the effect of eIF4G and eIF4B on the eIF4A conformational cycle. (A) In the presence of (poly-U) RNA and ATP, eIF4G (green) accelerates closing of eIF4A (gray) more than opening, and shifts the conformational equilibrium to the closed state. This effect is caused by altering the reference state (half-open), leading to a reduction in ΔG between (half-)open and closed state, and an increase in kclose. A concomitant reduction of the activation energy (green: with eIF4G, black: without eIF4G) increases kclose and kopen. (B) In the presence of (poly-U) RNA and ATP, eIF4B (red, in presence of eIF4G, green) predominantly accelerates closing, and shifts the conformational equilibrium to the closed state. The selective effect on kclose can be explained by an increased energy level of the half-open state that reduces the activation barrier (red: with eIF4B, black: without eIF4B) for closing only, but not for opening. The ΔG for closing is reduced, and the conformational equilibrium is altered by an increase in Kclose. (C) Individual steps in a productive conformational cycle of eIF4A in the presence of eIF4G and eIF4B, and rate constants for opening and closing determined from dwell time histograms. In the nucleotide-free and ADP states, at the beginning and the end of the conformational cycle, eIF4A is in the half-open state (yellow) when eIF4G is bound. In the ATP-bound state, the hydrolysis- and unwinding competent activated state (ATP*), and the ADP·Pi-state, eIF4A is in the closed conformation (orange). The rate constants determined from smFRET dwell times, kopen and kclose, are the rate constants for processes leading away from the closed and open states, respectively, and are dominated by the rate-limiting step in the relevant sequence of the catalytic cycle. The red and green arrows indicate the effects of eIF4B (red) and eIF4G (green) on opening and closing rates. For simplification, unproductive cycles with ATP hydrolysis in the absence of unwinding are not shown.
