Figure 2.
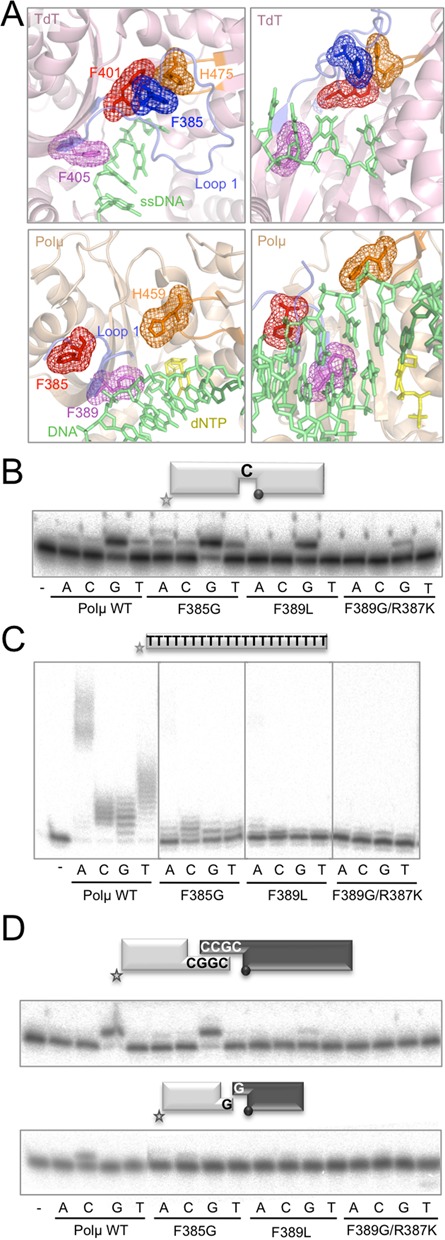
Relationship between TdT activity and NHEJ efficiency: single mutations in Loop1 affecting its structure/function. (A) Cartoon representations of the structures of murine TdT bound to ssDNA (1KDH, light pink) and the murine Polμ ternary complex (2IHM, wheat), showing the Loop1 in a blue cartoon and selected residues in sticks and mesh. Numbering of Polμ residues corresponds to the human enzyme, for congruence with the numbering used throughout the text. DNA substrate is shown in green and incoming nucleotide in yellow. (B) Gap-filling reactions were performed as described in the Materials and Methods section with the indicated proteins (25 nM) using a gaped substrate containing the oligonucleotides SP1C, T13C and DG1-P. When indicated, dNTPs were added separately at 10 nM in the presence of 2.5-mM MgCl2. (C) Terminal transferase activity assay with the indicated proteins (600 nM) using a homopolymeric substrate (polydT) and each of the four dNTPs (100 μM). Reactions were incubated for 30 min at 37ºC. (D) NHEJ reactions were performed with 200 nM of the indicated proteins and using four sets of substrates: the labelled substrates were formed by hybridization of 1G with 1D-NHEJ or D3-C with D1, and the cold substrates by hybridization of either 2G with 2D-NHEJ or D4-C with D2. The gray spheres indicate the presence of a 5′-P group in the downstream strand of the substrate. When indicated, each of the four ddNTPs (10 μM) was added in the presence of 2.5-mM MgCl2.
