Figure 1.
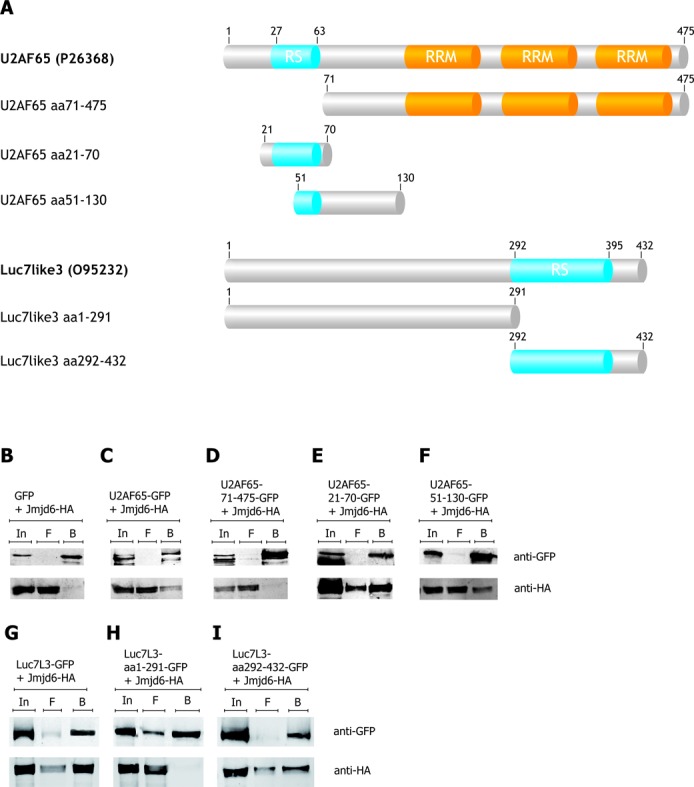
U2AF65 and Luc7L3 bind Jmjd6 via their RS domains. The U2AF65 protein exhibits two bona fide RNA binding domains (RRM) and a modified RRM, the so-called U2AF-homology motif (UHM), and an arginine–serine-rich (RS) domain (aa27–63). Various U2AF65 deletions and Luc7L3 variants were generated (A) and tested for Jmjd6 binding in co-immunoprecipitation assays (B–F). Full-length U2AF65 coupled to GFP and HA-tagged Jmjd6 were over-expressed in HEK 293T cells, lysed and immunoprecipitated with the GFP-nanotrap (32). Co-precipitation of HA-tagged Jmjd6 was tested in immunoblots with anti-HA antibody. Whereas the U2AF65-GFP co-precipitated Jmjd6-HA (C), the GFP-only control did not co-precipitate Jmjd6-HA (B). A U2AF65 lacking the RS-domain (U2AF65 71–465 GFP) did not interact with Jmjd6-HA (D). The RS-domain of U2AF65 (U2AF65 21–70 GFP) fused to GFP is sufficient to co-precipitate Jmjd6-HA (E). A truncated RS-domain (U2AF65 51–130 GFP) also interacted with Jmjd6-HA (F). A Luc7L3 variant lacking the RS-domain (Luc7L3 1–291 GFP) did not interact with Jmjd6-HA (H). But full-length Luc7L3-GFP (G) and the RS-domain of Luc7L3 fused to GFP (Luc7L3 292–432 GFP) are sufficient to co-precipitate Jmjd6-HA (I). In = input, F = flow-through, B = beads.
