Figure 2.
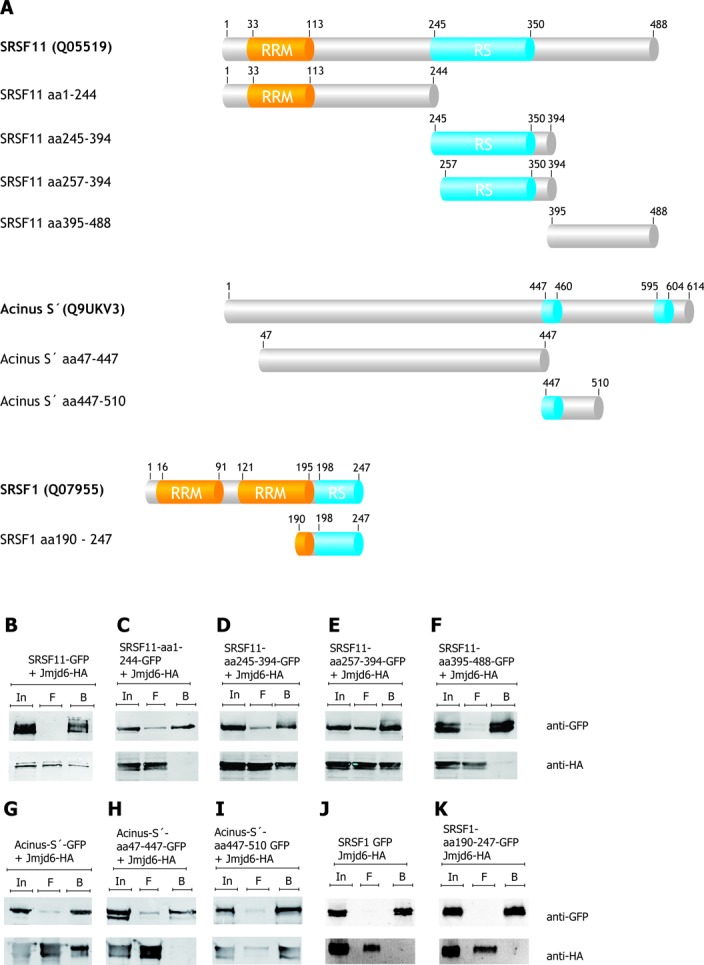
Acinus S′ and SRSF11, but not SRSF1 bind Jmjd6 via their RS domains. The domain structure of full-length Acinus S′, SRSF11, SRSF1 and deletions as investigated are shown schematically (A). GFP-tagged proteins were over-expressed with HA-tagged Jmjd6 in HEK 293T cells, lysed and immunoprecipitated with the GFP-trap (32). The SRSF11 protein fused to GFP co-precipitated Jmjd6-HA (B). The RS domain of SRSF11 is also sufficient to interact with Jmjd6-HA (D, E). SRSF11 variants lacking the RS domain did not interact with Jmjd6-HA (C, F). Acinus S′-GFP co-precipitated Jmjd6-HA (G), but not the truncated Acinus S′ version, lacking the RS domains (Acinus S′ 47–447 GFP) (H). The first RS domain (Acinus S′ 447–510 GFP) is sufficient to pull down Jmjd6-HA (I). The ‘classical’ SR–protein SRSF1 (37) exhibits an RS domain, but did not interact with Jmjd6-HA in our experiments (J). Fusing the RS-domain of SRSF1 to GFP also did not pull down Jmjd6-HA (K). In = input, F = flow-through, B = beads.
