Figure 8.
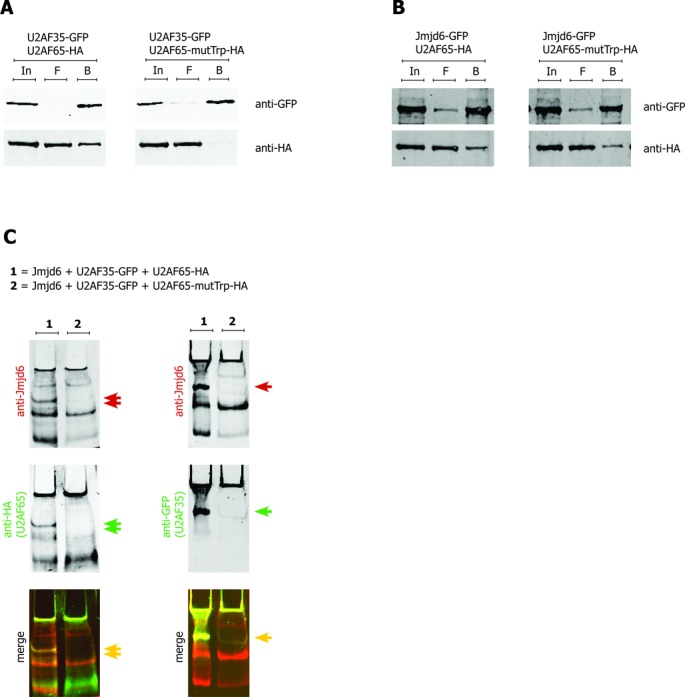
U2AF65–U2AF35 interaction is essential for Jmjd6 complex formation. Interaction of the U2AF-heterodimer is based on recognition of a specific tryptophane finger motif in U2AF65 by a modified RRM of U2AF35 (45). Mutation of the U2AF35 binding site in U2AF65 (U2AF65-mutTrp: W92A, P96G, P104G) inhibited the co-immunoprecipitation of HA-tagged U2AF65-mutTrp by GFP-tagged U2AF35 (A). In contrast, GFP-tagged Jmjd6 is able to pull down both, wildtype and mutTrp U2AF65 (B). Lysates of 293T cells overexpressing Jmjd6 (pcDNA3), U2AF35-GFP and either wildtype HA-tagged U2AF65 (lane 1) or HA-tagged U2AF65-mutTrp (lane 2) were separated on a native gel (C). As in Figure 7 two bands show co-migration of Jmjd6 with U2AF65 (red, green and yellow double arrows, left hand panel. (1) The slower migrating band contains U2AF35 (right hand panel, 1). With U2AF65-mutTrp the U2AF35-containing band is not present (both panels, (2) a trimeric U2AF65–U2AF35–Jmjd6 complex is not observed with U2AF65-mutTrp.
