Abstract
DRG is of importance in relaying painful stimulation to the higher pain centers and therefore could be a crucial target for early intervention aimed at suppressing primary afferent stimulation. Complex regional pain syndrome (CRPS) is a common pain condition with an unknown etiology. Recently added new information enriches our understanding of CRPS pathophysiology. Researches on genetics, biogenic amines, neurotransmitters, and mechanisms of pain modulation, central sensitization, and autonomic functions in CRPS revealed various abnormalities indicating that multiple factors and mechanisms are involved in the pathogenesis of CRPS. Epigenetics refers to mitotically and meiotically heritable changes in gene expression that do not affect the DNA sequence. As epigenetic modifications potentially play an important role in inflammatory cytokine metabolism, neurotransmitter responsiveness, and analgesic sensitivity, they are likely key factors in the development of chronic pain. In this dyad review series, we systematically examine the nerve injury-related changes in the neurological system and their contribution to CRPS. In this part, we first reviewed and summarized the role of neural sensitization in DRG neurons in performing function in the context of pain processing. Particular emphasis is placed on the cellular and molecular changes after nerve injury as well as different models of inflammatory and neuropathic pain. These were considered as the potential molecular bases that underlie nerve injury-associated pathogenesis of CRPS.
MeSH Keywords: Chronic Pain, Complex Regional Pain Syndromes, Epigenomics, Ganglia, Spinal, Peripheral Nerve Injuries
Background
Complex regional pain syndrome (CRPS) is a more recent concept developed from the original name “causalgia” and “Sudeck’s dystrophy”, and defined as the painful limb-confined condition by the International Association for the Study of Pain (IASP) in 1993 [1]. The prevalence of CRPS ranged from 5 to 26/100,000 per life-year [2]. For CRPS patients, 45 percent of them had nerve lesion, and around 40 percent had preceding tissue trauma, and 10 percent had only minor trauma, and 5 percent were spontaneously [2]. However, no distinctive correlation was found between the severity of injury and the degree of CRPS symptoms [1]. Inflammatory response was considered as one of the underlying contributors to the development and maintenance of CRPS, whereas the pharmacologic evidence using anti-inflammatory therapies on pro-inflammatory mediators was limited due to the trial quality, sample size and inconsistent reporting on the results [3]. Besides, the enhanced autonomic sympathetic activity was found facilitating nociceptive fibers to develop CRPS, but no direct evidence of activation of nociceptors related to sympathetic discharge was found [4]. Therefore, central nerve system (CNS) was concentrated on for its involvement in the pathogenesis of CRPS, especially when nerve injury existed [2,5]. In general, pain signal produced from the distal area due to various reasons needs to be transmitted along afferent fibers to dorsal root ganglia (DRG), the first relay station for pain signaling into the CNS. From this, DRG is considered as a critical pathogenic contributor to pain and then a potential analgesic target [6].
Many mediators like neurotransmitters, ionic channels and cytokines have been identified being involved in the regulation of pain through functioning on the DRG neurons. However, the therapeutic effect is limited when focusing on these factors, especially in the in vivo translational settings. Although the peripheral nervous system (PNS) is the major location of most currently available local analgesics [7] particularly the DRG for nerve injury-induced neuropathic pain [6], unfortunately, clinicians still only depend on ineffective drugs for pain control. Furthermore, only few basic research discoveries have been translated from animal pain models into clinical pain therapy [8]. The major reason for the inconsistency between robust basic reports and rare effective clinical translation is that we are still unclear about the real etiology of pain, especially when acute pain chronified after nerve injury.
In the past decades, four major categories of mediators were identified contributing to the development of pain: neurotransmitters, cytokines, endocrine and immune-mediators, and second messengers and nuclear mediators [9]. No matter where these mediators are located – DRG, spinal cord or brain, their contents and expressions were determined by corresponding levels of gene transcription and translation that largely depend on the modification and controlled by epigenetics [10]. As the entrance site of pain transmitted into the CNS, DRG possesses a pivotal role in the functional regulation of all those above-mentioned mediators in the context of chronic pain. This dyad review includes two parts. The first part (Part I) is mainly focused on nerve injury-related alterations on the molecular and cellular bases, and in the second part (Part II), nerve injury-induced activation of various singnaling pathways and epigenetic etiology will be discussed.
Nerve Injury and the Pathogenesis of CRPS
In terms of the pathologic causes of CRPS, two types of geneses exist: non-nerve injury and nerve injury that result in types 1 and 2 CRPS, respectively. Although a definable nerve lesion can be tracked for the type 2, it is still difficult for clinicians to figure out the difference accurately between the two types and diagnose patients following the entry criteria. In fact, CRPS, like the term itself is a complex pathologic state with the manifestations overlaid and mingled. One thing we do not know is whether the changes like edema, skin color, temperature and regional inflammatory responses can reflect or cause lesion of peripheral or terminal nerve fibers.
As far as the nerve injury we know, it is a wide-spectrum definition indicating the injury of the nerve tissue. Three major types of injury demonstrate different levels of lesion on the nerve fibers: level I, neurapraxia, the least severe and reversible injury from compression of the nerve or disruption of the blood supply without structural changes; level II, axonotmesis, a more severe injury due to crush or stretch resulting in axon damage with intact myelin sheath; and level III, neurotmesis, the most severe and even transectional injury leading to loss of nerve continuity [11]. Theoretically, nerve injury would results in loss of superficial sensation including pain (anesthesia, hyposthesia, parathesia), but on the contrary the fact is that nerve injury produces more severe pain [12]. Studies on proximal nerve fibers showed that the density, structure and function of these fibers encountered alteration in the context of CRPS [13] suggesting that nerve lesion was either the cause or the result of CRPS. Meanwhile, it indicated that nerve lesion is a critical contributor to the occurrence of CRPS whichever type of the pathologies the patient suffered. Therefore, the injury on nerve fibers in CRPS can be resulted from both internal (indirect lesion subsequent to tissue injury-induced inflammation) and external (direct trauma) causes.
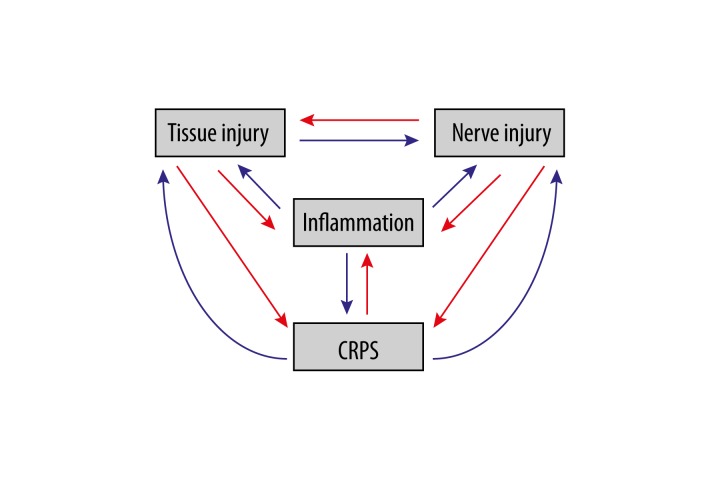
CRPS itself appears to be the final result of all kinds of pathological injuries. Is it the fact? Currently, almost no studies there paid attention to this question. When a patient was diagnosed CRPS, which means this patient had a “complex” pathologic condition where tissue injury, inflammation and nerve injury perform function on each other in a positive feedback manner that results in the so-called regional pain dubbed as a “syndrome” (Figure 1).
Figure 1.
Complex connections among factors in CRPS.
Nerve Injury Induced Cellular and Molecular Changes
When nociceptive signals produced at distal nerve fibers, and travelled through DRG neurons and then conveyed centrally into the CNS, the whole pain coding and transducing processes completed within several seconds even shorter. Following nerve injury, DRG neurons become hyperexcitable, generating spontaneous action potentials (APs) or abnormal high-frequency activity (especially when the injury lasts longer from several weeks to months) that substantially contributes to neuropathic pain. The development of chronic pain was considered as a mixed result of multiple factors needing the involvement of neurons, immune cells and glia, whereas neurons are still the core and immuno- and glial cells are only the supplemental contributors to the establishment of chronic pain. We hereby merely focus on DRG neurons for its role in the formation of nerve injury associated pain, and review the association between the DRG neuronal changes after nerve injury and CRPS.
Ion Channels and Nerve Injury
Ionic channels are pivotal contributors to the pain. Sodium channels are the major structural component of the formation of APs, and also are the functional target of local anesthetics. Voltage-gated sodium channels (Nav) on the DRG neurons play a crucial role in contributing to the development and maintenance of chronic pain [14]. According to the alpha subunit of Nav channels, a total of seven Nav channels thus far were found to be involved in different pain conditions. Potassium channels, the most widely distributed type of ion channels, were found to be involved in the regulation of pain. Voltage-gated potassium channel 1.1 (Kv1.1) acts as a mechanosensitive brake that regulates mechanical sensitivity of fibers related to mechanical perception [15]. Kv7/M channels expressed largely in DRG neurons take an important part in the nociceptive modulation and in the analgesic action of celecoxib, a widely prescribed cyclooxygenase 2 inhibitor [16]. Kv9.1 dysfunction or down-regulation leads to spontaneous and evoked neuronal hyperexcitability in myelinated fibers and DRG neurons, coupled with development of neuropathic pain [17]. The role of T-type calcium channels in sensory and nociceptive pathways has been well documented [18]. Cation-chloride cotransporters have been considered to play an essential role in controlling intracellular chloride concentration ([Cl−]i) of neurons, and this action hence produces modulation on the GABAergic function [19]. These major ionic and cationic channels function on each other and form a balancing matrix that controls over the final sensitivity of the DRG neurons.
Neurotransmitters and Nerve Injury
Alterations in the expression of neurotransmitters and corresponding receptors play a significant role in sensitizing or desensitizing DRG neurons after nerve injury. The imbalanced levels of excitatory and inhibitory transmitters including their receptors make the whole DRG be easier to be sensitized to stimulation depicting a reduced threshold or spontaneous activity. Meanwhile, nerve injury induced changes in the synaptic plasticity and homeostasis of the neural micro-environment giving way to hypersensitivity. These are the underlying reasons resulting in the complex refractory CRPS.
Glutamatergic Transmission After Nerve Injury
Glutamate is the great majority of excitatory neurotransmitter in our nervous system. Cumulating evidence indicated that glutamatergic transmission contributes to the pathogenesis and pathophysiology of pain allowing for an increasing approach to drug development. From its synthesis to release to reuptake, the contents of glutamate are kept in a reasonable level. Nerve injury, one of the major pathogenic reasons, will overbalance the glutamatergic homeostasis and will lead to hypersensitivity. As the metabolic precursor to gamma-aminobutyric acid (GABA), the level of glutamate elevated considerably due to the dysfunction of glutamic acid decarboxylase 65 (GAD65) under the condition of nerve injury [20]. Before released into the cleft, cytosolic glutamate first need cross the vesicular membrane via the activity of vesicular glutamate transporters (VGLUTs), but interestingly the expression of VGLUTs up-regulated in DRG neurons after peripheral nerve injury [21] and the vesicular VGLUT content was critically essential for the quantal size and the VGLUT2-mediated reduction of excitation affects sensory processing [22] suggesting that VGLUT-associated increase in the glutamate release was an another important contributor to the elevated glutamatergic transmission.
Once released into the cleft, glutamate needs bind to its receptors for further function. Ionotropic glutamate receptor (iGluR) including N-methyl-D-aspartate (NMDA), kainate and 2-amino-3-[3-hydroxy-5-methyl-isoxazol-4-yl]propanoic acid (AMPA) receptors forms the ion channel pore when glutamate binds to the receptor, but metabotropic glutamate receptor (mGluR) indirectly activates ion channels through a G-protein coupled signaling cascade. The expression of NMDA receptors (NRs) in DRG neurons was confirmed using double fluorescence staining and was involved in the regulation of the release of substance P (SP) from primary afferent nociceptors [23]. In later 1970s, DRG neurons were found to be depolarized by kainate, and peripheral administration of kainate receptor agonists elicited nociceptive signaling followed by pain behaviors [24]. Electrophysiological studies indicated that kainate receptors are located both postsynaptically (mainly mediating excitatory neurotransmission) and presynaptically (modulating excitatory and inhibitory transmissions). Activation of presynaptic AMPA receptors caused inhibition of glutamate release from the terminals showing that primary afferent depolarization is also mediated by glutamate acting on presynaptically localized AMPA receptors [25]. Although current evidence demonstrated that glutamate-mediated DRG neuronal activation is an important component of pain subsequent to nerve injury, the exact mechanisms are still not well understood.
GABAergic Transmission After Nerve Injury
GABA functions as the chief inhibitory transmitter through binding to its specific receptors resulting in negative change in the transmembrane potential. A number of data demonstrated that both subtypes of GABA receptors (GABAA and GABAB) in the DRG neurons take part in the pathologic processes of pain due to nerve injury, and inhibition in GABAergic transmission was considered as the underlying reason. Nerve injury induced changes in the expression of the GABA receptors, and structural remodeling, and GABA metabolism abnormality and function reduction all make the DRG neurons easier to be excited in response to stimulation.
Regarding the expression of GABA receptors in the DRG after nerve injury, different results reported. Some found a down-regulation of GABAA γ2 subunit [26], but some showed an up-regulation of GABAA α5 subunit [27] in ipsilateral DRG neurons after nerve injury. No matter the expression of GABAA receptor subunits and their precise role in pain modulation, one thing confirmed is that direct use of muscimol, a specific GABAA agonist, to the ipsilateral DRG prevented the development of hyperalgesia in rats subjected to sciatic nerve injury. GABAB was found to be positioned presynaptically modulating primary afferent transmission, and loss of GABAB on DRG neurons contributes to the development of hyperalgesia after nerve ligation [28]. Blockade of GABAB with pertussis toxin prevented the increase in the Nav1.7 protein that characterized the regulation of the phenotype of the DRG and primary afferent [29]. In addition, GABAA receptor associated protein (GABARAP) functions as an important component of the transient receptor potential vanilloid (TRPV1) signaling complex that contributes to the desensitization of DRG neurons [30]. Introducing recombinant adeno-associated virus 2 (rAAV2)-GAD65 using transgene delivery to the DRG increased the contents of GABA and consequently relieved pain from sciatic nerve injury [31]. Based on these findings, timely enhancement of the GABAergic transmission at the level of DRG can prevent the occurrence and development of hypersensitivity and may promote early recovery form peripheral nerve injury.
Monoaminergic Transmission After Nerve Injury
Monoaminergic neurotransmitters like dopamine (DA), norepinephrine (NE) and serotonin (5-HT) are critical components of neural activity modulation. When they are released into synaptic cleft through specific transporters, their levels are balanced by corresponding reuptake process. In the CNS, both ascending and descending pathways are composed. In term of the origination of these pathways, serotonergic fibers come from raphe nuclei, and noradrenergic projections stem from locus coeruleus (LC), and dopaminergic fibers derived majorly from ventral tegmental area (VTA) and substantia nigra (SN). The projections of these pathways are disturbed by nerve injury, and their function overlay and double or triple reuptake inhibitors are recommended in the relief of pain. Tetrahydrobiopterin (BH4), an essential co-factor in the production of monoamines, and its synthesis cascade were considered as targets of anti-neuropathic pain drug development.
Dopaminergic Transmission
By focusing on the two brain dopaminergic pathways, compelling evidence suggested a crucial role for dopamine neurotransmission in modulating pain perception and analgesia. In developing brain, the glutamatergic phenotype of the VTA DA neurons is highly plastic, repressed toward the end of normal embryonic development, and derepressed postnatally following injury indicating that glutamate and DA are co-released by mesencephalic neurons [32]. Unilateral chronic constriction injury (CCI) of the sciatic nerve induced specific and lateralized adaptations in the dopaminergic circuitry of the nucleus accumbens (NAcc) [33]. Activation of D2-like dopamine receptor or inhibition of D1-like receptor in the ventrolateral orbital cortex (VLO) produced anti-hypersensitivity effect [34]. Moreover, activation of the striatal dopamine D2 receptors attenuated neuropathic hypersensitivity via the suppression of impulse discharge of presumably pronociceptive neurons in the rostroventromedial medulla [35]. Reduction in mu-opioid receptor (MOR) function and in extracellular signal-regulated kinase (ERK) activity of dopaminergic neurons in the VTA contributed to the suppression of the morphine-induced rewarding effect under a neuropathic pain condition [36]. All these studies observed the dopaminergic contribution to the nerve injury evoked pain, but they only focused on the brain ascending dopaminergic pathways but not on the descending pathway. Meanwhile, what the potential effect of DRG dopamine transmission after nerve injury still needs to be investigated.
Noradrenergic Transmission
NE is released from postganglionic neurons in the sympathetic nervous system. Recent evidence showed that majority of noradrenergic fibers derived from the LC exert inhibitory and facilitating function on pain modulation. Intrathecal application of NE or clonidine produced antinociceptive effects by inhibiting Aδ and C-fiber-mediated sensory transmission through activating a2-adrenoceptors (ARs) indicating that descending noradrenergic projections to the spinal cord characterized as an inhibitory control over the pain. In contrast to this, lesion of noradrenergic LC markedly reduced tonic behavioral responses to peripheral inflammatory injury produced the idea that the LC might contribute to the induction or/and maintenance of hyperalgesia in the context of nerve injury-induced pain [37]. Furthermore, inhibition of the catechol-O-methyltransferase (COMT), an enzyme that metabolizes catecholamines, resulted in an increase in pain sensitivity through β2/3-adrenergic activation suggesting that β2/3 AR antagonists may benefit patients suffering from pain [38]. For the role of DRG noradrenergic transmission in pain, studies found that NE enhanced the excitability of DRG neurons via a2-ARs activation indicating that NE plays a pivotal role in the development and persistence of pain by exciting a2-ARs located on DRG neurons [39]. Therefore, we can conclude from recent findings that activation of different subtypes of noradrenergic receptors at different sites of the CNS produces contrasting results under the condition of pain.
Serotonergic Transmission
Like noradrenergic transmission, activation of the serotonergic pathway also possesses bidirectional effect on the modulation of pain. On the one hand, the descending 5-HT from the rostral ventromedial medulla (RVM) functioned as an essential contributor to pain facilitation during the development of persistent pain. Activation of the spinal 5-HT2A receptors produced a pro-nociceptive role, and spinal 5-HT3 receptor activation induced behavioral hypersensitivity via a neuronal-glial-neuronal signaling cascade [40]. In further, 5-HT increased the activity of capsaicin-sensitive peripheral nociceptors, which can be attenuated by pharmacologically blocking peripheral 5-HT receptors. These results suggest a facilitating role of the descending 5-HT in the development of persistent pain after tissue and nerve injury. However, on the other hand, 5-HT could enhance the release of GABA and glycine by activating the 5-HT2A, 5-HT2C and/or 5-HT3 receptors expressed on inhibitory spinal interneurons to inhibit sensory transmission [41], and the inhibitory descending serotonergic systems may be involved, through 5-HT1A and 5-HT3 receptors, in the production of an anti-nociceptive effect [42]. As thus an inhibitory effect of the serotonergic transmission exists on the nerve injury-induced pain.
For the DRG neurons, 5-HT also has dual effect on pain modulation. 5-HT can increase hyperpolarization-activated cation current (IH) especially the cells with T-type Ca2+ current amplitude and large-diameter DRG cells suggesting that 5-HT-induced changes in IH possibly be involved in the modulation the ectopic spontaneous discharges of DRG neurons subsequent to peripheral nerve injury [43]. On the contrary, enhanced serotonergic transmission stimulated secretion of oxytocin (OXT) [44], a molecule was found to be involved in the anti-nociceptive regulation through acting on vasopressin-1A receptor (V1AR) expressed in DRG neurons. So, further studies are needed to decipher the exact role of serotonergic system in pain subsequent to nerve injury.
Opioidergic Transmission After Nerve Injury
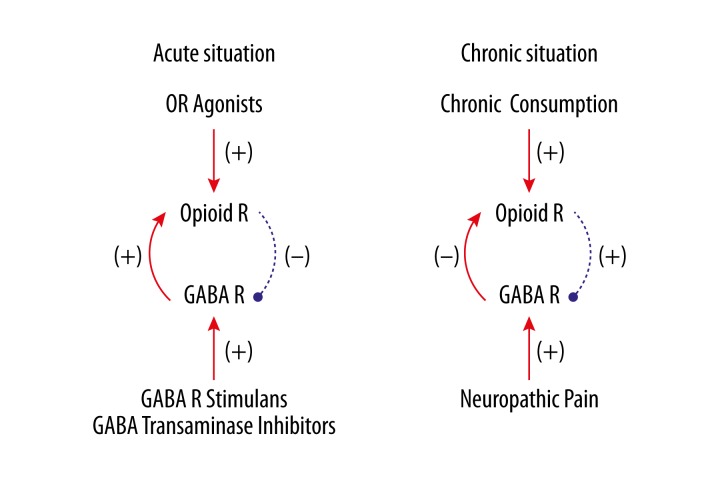
Opioid is still the mainstay of pain control, although great progress has made in the study of analgesia and other types of analgesics were developed. Exogenous opioid functions through binding to opioid receptors (ORs), a group of G-protein coupled receptors functioning as the bases of the endogenous opioidergic system, which plays an important role in the balance regulation of pain by mingling with other systems like GABAergic, noradrenergic, serotonergic, dopaminergic and cholinergic in both peripheral and central nervous systems [45]. GABA receptor stimulants and GABA-transaminase inhibitors enhance the analgesic effect of opiates; on the contrary, mu-opioid receptor (MOR) activation inhibits presynaptic GABA release, and this effect can be suppressed by CCK-8S, an endogenous anti-opioid substance. Chronic morphine consumption increases GABA synaptic activity that consequently leads to morphine tolerance [46]. The endogenous opioidergic analgesia was inhibited by increased presynaptic GABA release in the periaqueductal gray (PAG) under neuropathic pain. Taken together, acute and chronic activation of both opioidergic and GABAergic systems produce completely opposite effects on each other (Figure 2).
Figure 2.
Interaction between opioid and GABA systems. Activation of opioidergic and GABAergic systems produce opposite effect on each other under acute (A) and chronic (B) circumstances. OR: opioid receptor; GABA: γ-aminobutyric acid; R: receptor.
Adrenergic and serotonergic fibers in the spinal cord form two neurochemically different descending inhibitory systems, and most of the adrenergic fibers are succeeded by the propriospinal opioidergic neurons while a few of the serotonergic fibers have such a succession. On the one hand, opioidergic activation itself is involved in the antinociceptive effect of 5-HT or serotonin reuptake inhibitor. For the cortical descending control, opioidergic and serotonergic systems synergically inhibit hypersensitivity under different pain conditions, and the interaction of both systems functions as the underlying mechanism of many analgesic substances like oleanolic acid, gabapentin, eugenol, and paroxetine [47], acupuncture [48], and high-intensity extended exercise [49]. On the other hand, opioid interacts with α2-adrenoceptors showing higher affinity for the α2B and α2C than for the α2A subtype in brain [50]. In the spinal cord, α2A is not directly involved in morphine analgesia, and even antagonists of α2A augment the antinociceptive actions of partial opioid agonists, but α2C favors the spinal antinociceptive and synergic effect of opioid. In DRG, the analgesic effect of α2 agonist can be enhanced by systemic daily morphine via up-regulation of the α2A, α2B and α2C ARs [51]. Meanwhile, β2 AR agonists potentially offer an antinociceptive role in the treatment of neuropathic pain by implicating the endogenous opioidergic system [52]. Collectively, a functional connection formed among opioids, 5-HT and noradrenalin by affecting each other, but the precise relationship still needs to be clarified.
There is a tight connection between the rostromedial tegmental nucleus (RMTg) and the VTA/SN which is mediated by dense, opioid-sensitive GABA innervations suggesting that the RMTg is a key structure in the MOR-dependent regulation of dopamine neurons. Activation of dopamine D2 receptors induces analgesia through involving a MOR related opioidergic link, and D2 receptor agonists potentiate opioid-induced antinociception. Besides, cholinergic transmission activates endogenous opioidergic interneurons, which inhibits GABAergic neurons that finally are associated with antinociception in the hippocampus [53] and the amygdala [54]. So we can conclude that the interaction of opioidergic with dopaminergic or with cholinergic needs the modulating involvement of GABAergic neurons.
Gaseous Mediators Being Involved in Pain Modulation After Nerve Injury
Hydrogen sulfide (H2S) is an endogenous gaseotransmitter synthesized by the endogenous enzymes cystathionine-β-synthetase (CBS) and cystathionine-γ-lyase (CSE). Recent data showed that H2S functions as a signaling molecule in the enteric nervous system, and emerging evidence indicated that H2S is a critical mediator of pain. Most data showed that H2S is a pro-nociceptive molecule. H2S participates in formalin-induced nociception in diabetic and non-diabetic rats, but H2S levels were decreased in sciatic nerve, DRG and spinal cord during diabetes-associated peripheral neuropathy development [55]. In addition, H2S increases excitability through suppression of sustained potassium channel currents of trigeminal ganglion neurons [56]. Spinal and peripheral application of NaHS, an H2S donor, sensitizes Cav3.2 T-type Ca2+ channels expressed in the DRG and spinal nociceptive neurons which lead to hyperalgesia and such an H2S-mediated potentiation of T-type Ca2+ channels will result in enhanced neuronal firing in concert to depolarization of TRPA1 in nociceptive sensory neurons. These findings were further convinced that mechanical hyperalgesia and allodynia induced by NaHS/H2S require activation of both Cav3.2 and TRPA1 channels [57]. In contrast to its pro-nociceptive role, H2S was also found exert antinociception in a rodent model of visceral pain by transactivating the MORs [58]. As thus, H2S possesses pro- and anti-nociceptive effects under different pain settings.
Nitric oxide (NO) is a gaseous signaling molecule synthesized by nitric oxide synthases (NOSs). New evidence showed that the NOS/NO pathway plays an important role in the development and maintenance of neuropathic pain after nerve injury. For the peripheral sensory neurons, the nociceptive action of NO is mediated by both TRPV1 and TRPA1 [59]. NO can down-regulate the function of TRPV1 through activation of the cGMP-PKG pathway, which participates in the local anti-allodynic effects of morphine after peripheral nerve injury, and NO is implicated in the down-regulation of MOR during neuropathic pain [60]. For the spinal cord, the NO-dependent long-term potentiation (LTP) of nociceptive pathways is a crucial mechanism behind long-term central pain sensitization [61]. Therefore, NO functions as a sensitizer of peripheral and spinal nociceptive neurons, but at the early stage of inflammatory pain, NO plays as an analgesic in the periphery.
Carbon monoxide (CO), an atypical gaseous neurotransmitter in the nervous system, has been found to be involved in a wide variety of neuronal activities including in nociception processing. Chronic pharmacological activation of heme oxygenase (HO)-1/CO pathway may prevent the development of behavioral symptoms of neuropathic pain, through an activation of anti-inflammatory and anti-oxidant mechanisms [62]. In further, HO-CO-cGMP pathway plays a key phasic antinociceptive role modulating noninflammatory acute pain and inflammatory pain, and an antinociceptive synergy exists between peripheral and spinal HO-CO pathways [63]. Application of both carbon monoxide-releasing molecule II (CORM-2) and heme oxygenase 1 inducer (cobalt protoporphyrin IX, CoPP) improved the local antinociceptive effects of morphine during chronic inflammatory and neuropathic pain by interaction with NO [64] and enhancing MOR peripheral expression and inhibiting spinal microglial activation and overexpression of NOS suggesting that an interaction between CO and NO systems is taking place following nerve injury.
Inflammation After Nerve Injury
Inflammatory responses subsequent to nerve injury play an essential role in defending further lesion and in promoting axon regeneration, but simultaneously contribute to the pathogenesis of hypersensitivity. As the natural consequence of nerve injury, inflammation was considered as one of the keys of the development and maintenance of pain, and also as the potential target of analgesia. For CRPS, the presence of a pro-inflammatory state is found to be an essential component contributing to its pathogenesis. There are important clinical implications for how to take advantage of nerve injury-induced inflammation to obtain a “double-win” effect, i.e. controlling inflammatory responses at a proper level to promote recovery from injury and suppress neural sensitivity.
Cytokines
Cytokines are small, nonstructural proteins functioning as the regulators of host responses to infection, immune responses, inflammation, and trauma. Functionally, cytokines are categorized into pro- and anti-inflammatory cytokines, which participate in balancing the inflammatory responses. Tumor necrosis factor (TNF) is a principal composition of pro-inflammatory cytokines. Emerging evidence indicated that TNF-induced inflammation is strongly associated with cellular harm and pain behavior, and also are bound by temporal and spatial resolutions [65]. TNF takes function by binding to its receptor-either TNF receptor type 1 (TNFR1) or TNFR2 or both. Majorly, three signaling cascades are activated by TNF: nuclear factor kappa B (NF-κB), mitogen-activated protein kinase (MAPK) family, and the death signaling. Of the MAPK cascades, TNF induces a strong activation of the stress-related c-Jun N-terminal kinase (JNK) group, evokes moderate response of the p38-MAPK, and is responsible for minimal activation of the classical ERK. TNF is involved in the sensitization of both peripheral and central neurons through inducing production of other inflammatory mediators, promoting overbalance between excitatory and inhibitory neurotransmitters, and facilitating plasticity of synaptic connection [66].
Macrophage migration inhibitory factor (MIF) is the first cytokine defined for its inhibition on the random migration of lymphocytes in 1960s’, and a number of data showed that MIF is a pluripotent regulator of inflammatory responses. Cumulating evidence indicated that MIF is a pivotal contributor to the pathogenesis of pain subsequent to nerve injury [67,68]. CD74 takes an essential part as the functional receptor of MIF in the mediation of downstream signaling pathway of MIF, and is considered as a promising therapeutic target of MIF-associated inflammatory responses. In consideration of MIF’s counter-regulation of glucocorticoids’ anti-inflammatory function [69], and the analgesic role of glucocorticoids [70], it is worthwhile for studying the correlation between MIF and glucocorticoids in pain, and the accurate role of MIF in the DRG neurons in the context of nerve injury associated CRPS also warrants further investigation.
Interleukin 1 (IL-1) is not only a product, but also an evoker of inflammation. Direct application of IL-1 into plantar caused hypersensitivity to mechanical stimulation, and IL-1 increased excitability of sensory neurons. Under the condition of pain, the levels of IL-1 mRNA, protein, and IL-1 receptors are up-regulated in nociceptors suggesting that IL-1 directly affects the excitability of primary afferents [71]. IL-1-induced inflammatory cascades make the sensory neurons be susceptible to subthreshold stimuli [72], and IL-1 functions as a key mediator in the interaction between glia and neurons [73] indicating that blockade of IL-1 or IL-1R possesses therapeutic property. IL-6 is another key mediator of inflammation. Emerging evidence indicates that IL-6 is strongly associated with nerve injury-induced hyperalgesia. Bilateral elevation of IL-6 protein and mRNA is not limited to DRG homonymous to the injured nerve, but also extended to DRG that are heteronymous to the injured nerve, which suggests that the neuroinflammatory reaction of DRG to nerve injury is propagated alongside the neuroaxis from the lumbar to the remote cervical segments. However, IL-6 exerts disinhibitory role in promoting axon regeneration after injury via janus kinase/signal transducers and activators of transcription-3 (JAK/STAT3) and phosphatidylinositide 3-kinase/protein kinase B (PI3K/Akt) pathways [74]. These imply that attenuation of nerve injury-induced pain by preventing the actions of TNF, MIF, IL-1, and IL-6 may be beneficial to the restoration of function of the impaired nerve.
Prostaglandins
Multiple lines of evidence indicate that eicosanoid signaling is crucial in initiation and progression of pain that have neuroinflammatory pathologic component. Spinal prostaglandin (PG) synthesis is enhanced by spinal nerve injury, and pharmacological disruption of this cascade significantly reverses such allodynia. Injured sciatic nerves produce PGE2 and 6-keto-PGF1α through COX-2 activity by macrophages in response to soluble factors [75]. In further, PGE2 participated in the maintenance of neuropathic pain in vivo not only by activating spinal neurons, but also by retaining microglia in the central terminals of primary afferent fibers via EP2 subtype and via EP1-mediated NO production, during which p38 MAPK activation is the underlying pathway[76]. Besides, PGE2 induced allodynia by stimulation of neuropeptide/opioid peptide nociceptin/orphanin FQ (N/OFQ) release in the spinal cord via PGE receptor 4 (EP4) subtypes [77]. Homeostasis of neuronal connection is an important property of the stable nervous system, but the inflammatory episodes after COX 2 is rapidly upregulated subsequent to nervous injury and its product PGE2 exerts contrast functions in the nervous system by working on the homeostatic plasticity, and new evidence revealed that COX2-PGE2 system takes an essential part in balancing excitation and inhibition of the synaptic activities at a new setpoint [78]. Therefore, targeting on PG for pain therapy promises patients as showed in the COX inhibitors in the treatment of pain related to inflammatory responses.
Others
Inflammation is orchestrated by means of the body’s immunity process and displayed as an intertwining connection among different types of inflammatory mediators. Except for the abovementioned factors being involved in the regulation of inflammation, there are other kinds of essential mediators contributing to the initiation and maintenance of inflammatory responses.
Kinins are a group of polypeptides produced at sites of injury and inflammation and serve an important role in signaling as well as organizing tissue distress and responsiveness to injury. Bradykinin (BK) and kallidin are two major components of kinin, and found to be involved in the regulation of a number of biochemical transduction mechanisms including vasodilatation, increased vascular permeability, the stimulation of immune cells and peptide-containing sensory neurons to induce pain. Several mechanisms have been proposed to account for hyperalgesia including the direct activation or sensitization of primary sensory neurons. Evidence indicated that BK B2 receptors are associated with acute pain state, but BK B1 receptors are responsible for the prolonged and maintenance of hyperalgesia [79]. At the primary afferent level, it is B2 but not B1 receptor mediates the sensitivity of C-fiber nociceptors to BK to a persistent inflammatory state [80]. Collectively, the utilization of kinin receptor antagonists is useful in alleviating CNS inflammation and in treating peripheral and central pain.
Substance P (SP) is a pivotal neurotransmitter and neurmodulator compound involving in the regulation of neurogenic inflammation to nerve injury by activating mast cells to release inflammatory mediators such as arachindonic acid compound, cytokines/chemokines and histamine. In rostroventromedial medulla (RVM)-spinal cord-projecting neurons, SP regulates the function of 5-HT and GABA. Interestingly, in the striatum, the activation of NK1R leads to pain suppression rather than facilitation, and volume transmission of SP appears to be indispensable for pain control suggesting that modulation of striatal NK1R could be a useful method of inducing analgesia [81]. Moreover, cocaine exposure and the knockdown of MOR affect the SP mRNA expression in zebrafish indicating that SP is connected with and affected by the opioidergic system [82]. SP also is involved in the sex differences in formalin-induced pain in males and females [83]. In the context of CRPS, SP was found to be a critical contributor to mast cell accumulation, degranulation, and sensitization through acting through the NK-1 receptor [84], to the vascular and nociceptive changes [85], and to the spontaneous extravasation, edema, warmth, and mechanical hyperalgesia [86]. Taken together, SP from different regions of the CNS functions differently in pain states by connecting with other modulating systems.
Histamine acts as a neurotransmitter and involved in the local immune responses as well as triggering the inflammatory response. Mast cells, the original source of histamine production play a key role in inflammation and the development of neuropathic pain. The H1 receptor-positive neurons were exclusively IB4 labeled ones, but not those with immunoreactive to SP and calcitonin gene-related peptide (CGRP) [87]. Selective blockades of H4 receptors and H3 receptors produce significant anti-nociception in inflammatory and neuropathic pain. A number of evidence underlined the critical role of histamine receptors in inflammation and its promising role as drug target in inflammatory diseases and nerve injury-associated pain states [88].
Hydrogen Ions determine the strength of acids, i.e. the pH value. Tissue acidosis is a common feature of many painful conditions. Tissue injury induces release of protons indeed among the first factors involving in depolarizing peripheral free terminals of nociceptors and leading to pain via reducing local pH value reached by the modulation of acid-sensing ion channels (ASICs) located principally in neurons. In sensory neurons, ASICs act as “chemo-electrical” transducers and are involved in the nociceptive regulation, and targeting ASICs at different levels of the nervous system was considered as a promising strategy for the relief of pain [89]. In consideration of the contribution of the up-regulated and overactivated ASICs to various forms of chronic inflammation and pain, ASICs and receptors are considered as targets for novel therapeutics [90].
Nerve growth factor (NGF) is crucial for the development and maintenance of sympathetic and sensory neurons in the nervous system, including nociceptors. A large number of evidence indicated that NGF is expressed at high levels in injured tissues, and in involved in the facilitation of pain transmission by nociceptive neurons in the context of acute and chronic pain. Intradermal application of NGF induces long-lasting axonal and mechanical sensitization in C nociceptors that corresponds to hyperalgesia, and prolonged injections of NGF into muscle produced a progressive manifestation of muscle soreness, mechanical hyperalgesia, temporal summation of pressure pain, and pressure-induced pain distribution [91]. Furthermore, inhibition of mature NGF degradation induces a sprouting of sympathetic fibers into the upper dermis of the skin that correlated with an increase in pain-related sensitivity [92]. In the context of diabetes neuropathy, NGF can reverse loss of the functional MOR on central terminals of sensory neurons [93], and NGF-TrkA signaling or NGF-p75 receptor inhibition protects somatic sensory neurons from viral protein exposure in HIV-induced neuropathy [94]. Overall, NGF is a critical mediator and modulator of pain. Due to its dual effects, detrimental and regenerative activities, NGF can be used as a potential therapeutic intervention of pain through applying either NGF antagonists or recombinant NGF.
Adenosine triphosphate (ATP) is a homo- and hetero-cellular neurotransmitter released from both neurons and astroglia, and the released ATP activates the ionotropic P2X receptors which regulate glia-driven modulation of fast synaptic transmission and synaptic plasticity. In the sensory nervous system, ATP is suggested to be one of first mediators of tissue damage, which activates primary afferents, and a multiple microglial P2Y metabotropic receptors activated by peripheral nerve injury play a key role in the development of neuropathic pain in the DRG and spinal cord [95]. Peripheral nerve entrapment increases ATP release in DRG neurons, and neuropathic injury causes DRG neurons to become hyperexcitable to ATP-evoked P2X receptor-mediated depolarization, a phenotypic switch sensitive to PKC modulation and mediated by increased activity of TTX-sensitive voltage-gated Na+ channels (VGSCs) [96]. Injury of DRG itself up-regulates functional P2X3 receptors suggesting that enhanced purinergic responses after chronic compression of DRG (CCD) are mediated by P2X3 receptors. Moreover, noradrenaline stimulates ATP release from DRG neurons as mediated via β3 adrenoceptors involving PKA activation to cause allodynia [97]. Currently, cumulating evidence convinced that purinergic neurotransmission, involving release of ATP as an efferent neurotransmitter functions in the peripheral and central nervous systems as a cotransmitter with glutamate, noradrenaline, GABA, Ach and dopamine [98].
Conclusions
CRPS as the name indicates is complex conditions with unclear pathology and unsolved underlying mechanisms. Neural sensitization refers to amplification of behavioral responses occurring following presentation of an aversive or noxious stimulus. Understanding the cellular and molecular underpinnings of the sensitization has been an overarching theme of nerve injury-induced pain. Focusing on these changes possesses pivotal value in seeking and discovering novel interventional targets for nerve injury-induced pain condition. These also are the bases of the next part of this review (Part II) to give further discussion on their downstream signaling cascades and epigenetic modifications on corresponding gene expression.
Footnotes
Conflict of Interests
None.
Source of support: This work is supported by the National Natural Scientific Foundation of China (NSFC, 30901397, 81271242, and 81371248); Nanjing Municipal Outstanding Young Scientist Grant in Medical Science Development (JQX12009); and Nanjing Municipal Developmental Key (ZKX10018) and Young (QYK11139) Grant of Medical Science
References
- 1.Stanton-Hicks M, Janig W, Hassenbusch S, et al. Reflex sympathetic dystrophy: changing concepts and taxonomy. Pain. 1995;63:127–33. doi: 10.1016/0304-3959(95)00110-E. [DOI] [PubMed] [Google Scholar]
- 2.Maihöfner C, Seifert F, Markovic K. Complex regional pain syndromes: new pathophysiological concepts and therapies. Eur J Neurol. 2010;17:649–60. doi: 10.1111/j.1468-1331.2010.02947.x. [DOI] [PubMed] [Google Scholar]
- 3.Parkitny L, McAuley JH, Di Pietro F, et al. Inflammation in complex regional pain syndrome: a systematic review and meta-analysis. Neurology. 2013;80:106–17. doi: 10.1212/WNL.0b013e31827b1aa1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Campero M, Bostock H, Baumann TK, Ochoa JL. A search for activation of C nociceptors by sympathetic fibers in complex regional pain syndrome. Clin Neurophysiol. 2010;121:1072–79. doi: 10.1016/j.clinph.2009.12.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ochoa JL, Verdugo RJ. Neuropathic pain syndrome displayed by malingerers. J Neuropsychiatry Clin Neurosci. 2010;22:278–86. doi: 10.1176/appi.neuropsych.22.3.278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sapunar D, Kostic S, Banozic A, Puljak L. Dorsal root ganglion – a potential new therapeutic target for neuropathic pain. J Pain Res. 2012;5:31–38. doi: 10.2147/JPR.S26603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Barreveld A, Witte J, Chahal H, et al. Preventive analgesia by local anesthetics: the reduction of postoperative pain by peripheral nerve blocks and intravenous drugs. Anesth Analg. 2013;116:1141–61. doi: 10.1213/ANE.0b013e318277a270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mogil JS. Animal models of pain: progress and challenges. Nat Rev Neurosci. 2009;10:283–94. doi: 10.1038/nrn2606. [DOI] [PubMed] [Google Scholar]
- 9.Wang F, Xu S, Shen X. Recent Development in Pain Research. 1st ed. Transworld Research Network; 2013. Epigenetic control of pain; pp. 1–18. [Google Scholar]
- 10.Teperino R, Lempradl A, Pospisilik JA. Bridging epigenomics and complex disease: the basics. Cell Mol Life Sci. 2013;70:1609–21. doi: 10.1007/s00018-013-1299-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Noaman HH. Surgical treatment of peripheral nerve injury. In: Rayegani SM, editor. Basic Principles of Peripheral Nerve Disorders. InTech; 2012. pp. 93–132. [Google Scholar]
- 12.Campbell JN. Nerve lesions and the generation of pain. Muscle Nerve. 2001;24(10):1261–73. doi: 10.1002/mus.1143. [DOI] [PubMed] [Google Scholar]
- 13.Oaklander AL, Fields HL. Is reflex sympathetic dystrophy/complex regional pain syndrome type I a small-fiber neuropathy? Ann Neurol. 2009;65:629–38. doi: 10.1002/ana.21692. [DOI] [PubMed] [Google Scholar]
- 14.Wang W, Gu J, Li YQ, Tao YX. Are voltage-gated sodium channels on the dorsal root ganglion involved in the development of neuropathic pain? Mol Pain. 2011;7:16. doi: 10.1186/1744-8069-7-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hao J, Padilla F, Dandonneau M, et al. Kv1.1 channels act as mechanical brake in the senses of touch and pain. Neuron. 2013;77:899–914. doi: 10.1016/j.neuron.2012.12.035. [DOI] [PubMed] [Google Scholar]
- 16.Mi Y, Zhang X, Zhang F, et al. The role of potassium channel activation in celecoxib-induced analgesic action. PLoS One. 2013;8:e54797. doi: 10.1371/journal.pone.0054797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Tsantoulas C, Zhu L, Shaifta Y, et al. Sensory neuron downregulation of the Kv9.1 potassium channel subunit mediates neuropathic pain following nerve injury. J Neurosci. 2012;32:17502–13. doi: 10.1523/JNEUROSCI.3561-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Todorovic SM, Jevtovic-Todorovic V. T-type voltage-gated calcium channels as targets for the development of novel pain therapies. Br J Pharmacol. 2011;163:484–95. doi: 10.1111/j.1476-5381.2011.01256.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Price TJ, Cervero F, de Koninck Y. Role of cation-chloride-cotransporters (CCC) in pain and hyperalgesia. Curr Top Med Chem. 2005;5:547–55. doi: 10.2174/1568026054367629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kim J, Kim SJ, Lee H, Chang JW. Effective neuropathic pain relief through sciatic nerve administration of GAD65-expressing rAAV2. Biochem Biophys Res Commun. 2009;388:73–78. doi: 10.1016/j.bbrc.2009.07.120. [DOI] [PubMed] [Google Scholar]
- 21.Malet M, Vieytes CA, Lundgren KH, et al. Transcript expression of vesicular glutamate transporters in lumbar dorsal root ganglia and the spinal cord of mice – Effects of peripheral axotomy or hindpaw inflammation. Neuroscience. 2013;248C:95–111. doi: 10.1016/j.neuroscience.2013.05.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Moechars D, Weston MC, Leo S, et al. Vesicular glutamate transporter VGLUT2 expression levels control quantal size and neuropathic pain. J Neurosci. 2006;26:12055–66. doi: 10.1523/JNEUROSCI.2556-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Liu H, Mantyh PW, Basbaum AI. NMDA-receptor regulation of substance P release from primary afferent nociceptors. Nature. 1997;386:721–24. doi: 10.1038/386721a0. [DOI] [PubMed] [Google Scholar]
- 24.Ault B, Hildebrand LM. Activation of nociceptive reflexes by peripheral kainate receptors. J Pharmacol Exp Ther. 1993;265:927–32. [PubMed] [Google Scholar]
- 25.Lee CJ, Bardoni R, Tong CK, et al. Functional expression of AMPA receptors on central terminals of rat dorsal root ganglion neurons and presynaptic inhibition of glutamate release. Neuron. 2002;35:135–46. doi: 10.1016/s0896-6273(02)00729-8. [DOI] [PubMed] [Google Scholar]
- 26.Obata K, Yamanaka H, Fukuoka T, et al. Contribution of injured and uninjured dorsal root ganglion neurons to pain behavior and the changes in gene expression following chronic constriction injury of the sciatic nerve in rats. Pain. 2003;101:65–77. doi: 10.1016/s0304-3959(02)00296-8. [DOI] [PubMed] [Google Scholar]
- 27.Xiao HS, Huang QH, Zhang FX, et al. Identification of gene expression profile of dorsal root ganglion in the rat peripheral axotomy model of neuropathic pain. Proc Natl Acad Sci USA. 2002;99:8360–65. doi: 10.1073/pnas.122231899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Engle MP, Merrill MA, Marquez De Prado B, Hammond DL. Spinal nerve ligation decreases γ-aminobutyric acidB receptors on specific populations of immunohistochemically identified neurons in L5 dorsal root ganglion of the rat. J Comp Neurol. 2012;520:1663–77. doi: 10.1002/cne.23005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Chattopadhyay M, Mata M, Fink DJ. Vector-mediated release of GABA attenuates pain-related behaviors and reduces Na(V)1.7 in DRG neurons. Eur J Pain. 2011;15:913–20. doi: 10.1016/j.ejpain.2011.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Laínez S, Valente P, Ontoria-Oviedo I, et al. GABAA receptor associated protein (GABARAP) modulates TRPV1 expression and channel function and desensitization. FASEB J. 2010;24:1958–70. doi: 10.1096/fj.09-151472. [DOI] [PubMed] [Google Scholar]
- 31.Kim J, Kim SJ, Lee H, Chang JW. Effective neuropathic pain relief through sciatic nerve administration of GAD65-expressing rAAV2. Biochem Biophys Res Commun. 2009;388:73–78. doi: 10.1016/j.bbrc.2009.07.120. [DOI] [PubMed] [Google Scholar]
- 32.Dal Bo G, Bérubé-Carrière N, Mendez JA, et al. Enhanced glutamatergic phenotype of mesencephalic dopamine neurons after neonatal 6-hydroxydopamine lesion. Neuroscience. 2008;156:59–70. doi: 10.1016/j.neuroscience.2008.07.032. [DOI] [PubMed] [Google Scholar]
- 33.Austin PJ, Beyer K, Bembrick AL, Keay KA. Peripheral nerve injury differentially regulates dopaminergic pathways in the nucleus accumbens of rats with either ‚pain alone’ or ‚pain and disability’. Neuroscience. 2010;171:329–43. doi: 10.1016/j.neuroscience.2010.08.040. [DOI] [PubMed] [Google Scholar]
- 34.Dang YH, Zhao Y, Xing B, et al. The role of dopamine receptors in ventrolateral orbital cortex-evoked anti-nociception in a rat model of neuropathic pain. Neuroscience. 2010;169:1872–80. doi: 10.1016/j.neuroscience.2010.06.050. [DOI] [PubMed] [Google Scholar]
- 35.Ansah OB, Leite-Almeida H, Wei H, Pertovaara A. Striatal dopamine D2 receptors attenuate neuropathic hypersensitivity in the rat. Exp Neurol. 2007;205:536–46. doi: 10.1016/j.expneurol.2007.03.010. [DOI] [PubMed] [Google Scholar]
- 36.Narita M, Suzuki M, Imai S, et al. Molecular mechanism of changes in the morphine-induced pharmacological actions under chronic pain-like state: suppression of dopaminergic transmission in the brain. Life Sci. 2004;74:2655–73. doi: 10.1016/j.lfs.2004.01.006. [DOI] [PubMed] [Google Scholar]
- 37.Decosterd I, Woolf CJ. Spared nerve injury: an animal model of persistent peripheral neuropathic pain. Pain. 2000;87:149–58. doi: 10.1016/S0304-3959(00)00276-1. [DOI] [PubMed] [Google Scholar]
- 38.Nackley AG, Tan KS, Fecho K, et al. Catechol-O-methyltransferase inhibition increases pain sensitivity through activation of both beta2- and beta3-adrenergic receptors. Pain. 2007;128:199–208. doi: 10.1016/j.pain.2006.09.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Tanimoto K, Takebayashi T, Kobayashi T, et al. Does norepinephrine influence pain behavior mediated by dorsal root ganglia?: a pilot study. Clin Orthop Relat Res. 2011;469:2568–76. doi: 10.1007/s11999-011-1798-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Gu M, Miyoshi K, Dubner R, et al. Spinal 5-HT(3) receptor activation induces behavioral hypersensitivity via a neuronal-glial-neuronal signaling cascade. J Neurosci. 2011;31:12823–36. doi: 10.1523/JNEUROSCI.1564-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 41.Xie DJ, Uta D, Feng PY, et al. Identification of 5-HT receptor subtypes enhancing inhibitory transmission in the rat spinal dorsal horn in vitro. Mol Pain. 2012;8:58. doi: 10.1186/1744-8069-8-58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Nakamoto H, Soeda Y, Seki T, et al. Possible involvement of descending serotonergic systems in antinociception by centrally administered elcatonin in mice. Biol Pharm Bull. 1999;22:691–97. doi: 10.1248/bpb.22.691. [DOI] [PubMed] [Google Scholar]
- 43.Cardenas CG, Mar LP, Vysokanov AV, et al. Serotonergic modulation of hyperpolarization-activated current in acutely isolated rat dorsal root ganglion neurons. J Physiol. 1999;518:507–23. doi: 10.1111/j.1469-7793.1999.0507p.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Saydoff JA, Rittenhouse PA, van de Kar LD, Brownfield MS. Enhanced serotonergic transmission stimulates oxytocin secretion in conscious male rats. J Pharmacol Exp Ther. 1991;257:95–99. [PubMed] [Google Scholar]
- 45.Vadivelu N, Mitra S, Hines RL. Peripheral opioid receptor agonists for analgesia: a comprehensive review. J Opioid Manag. 2011;7:55–68. doi: 10.5055/jom.2011.0049. [DOI] [PubMed] [Google Scholar]
- 46.Ma J, Pan ZZ. Contribution of brainstem GABA(A) synaptic transmission to morphine analgesic tolerance. Pain. 2006;122:163–73. doi: 10.1016/j.pain.2006.01.031. [DOI] [PubMed] [Google Scholar]
- 47.Duman EN, Kesim M, Kadioglu M, et al. Possible involvement of opioidergic and serotonergic mechanisms in antinociceptive effect of paroxetine in acute pain. J Pharmacol Sci. 2004;94:161–65. doi: 10.1254/jphs.94.161. [DOI] [PubMed] [Google Scholar]
- 48.Erthal V, da Silva MD, Cidral-Filho FJ, et al. ST36 laser acupuncture reduces pain-related behavior in rats: involvement of the opioidergic and serotonergic systems. Lasers Med Sci. 2013;28:1345–51. doi: 10.1007/s10103-012-1260-7. [DOI] [PubMed] [Google Scholar]
- 49.Mazzardo-Martins L, Martins DF, Marcon R, et al. High-intensity extended swimming exercise reduces pain-related behavior in mice: involvement of endogenous opioids and the serotonergic system. J Pain. 2010;11:1384–93. doi: 10.1016/j.jpain.2010.03.015. [DOI] [PubMed] [Google Scholar]
- 50.Höcker J, Böhm R, Meybohm P, et al. Interaction of morphine but not fentanyl with cerebral alpha2-adrenoceptors in alpha2-adrenoceptor knockout mice. J Pharm Pharmacol. 2009;61:901–10. doi: 10.1211/jpp/61.07.0009. [DOI] [PubMed] [Google Scholar]
- 51.Tamagaki S, Suzuki T, Hagihira S, et al. Systemic daily morphine enhances the analgesic effect of intrathecal dexmedetomidine via up-regulation of alpha 2 adrenergic receptor subtypes A, B and C in dorsal root ganglion and dorsal horn. J Pharm Pharmacol. 2010;62:1760–67. doi: 10.1111/j.2042-7158.2010.01192.x. [DOI] [PubMed] [Google Scholar]
- 52.Yalcin I, Tessier LH, Petit-Demoulière N, et al. Chronic treatment with agonists of beta(2)-adrenergic receptors in neuropathic pain. Exp Neurol. 2010;221:115–21. doi: 10.1016/j.expneurol.2009.10.008. [DOI] [PubMed] [Google Scholar]
- 53.Favaroni Mendes LA, Menescal-de-Oliveira L. Role of cholinergic, opioidergic and GABAergic neurotransmission of the dorsal hippocampus in the modulation of nociception in guinea pigs. Life Sci. 2008;83:644–50. doi: 10.1016/j.lfs.2008.09.006. [DOI] [PubMed] [Google Scholar]
- 54.Leite-Panissi CR, Brentegani MR, Menescal-de-Oliveira L. Cholinergic-opioidergic interaction in the central amygdala induces antinociception in the guinea pig. Braz J Med Biol Res. 2004;37:1571–79. doi: 10.1590/s0100-879x2004001000018. [DOI] [PubMed] [Google Scholar]
- 55.Velasco-Xolalpa ME, Barragán-Iglesias P, Roa-Coria JE, et al. Role of hydrogen sulfide in the pain processing of non-diabetic and diabetic rats. Neuroscience. 2013;250:786–97. doi: 10.1016/j.neuroscience.2013.06.053. [DOI] [PubMed] [Google Scholar]
- 56.Feng X, Zhou YL, Meng X, et al. Hydrogen sulfide increases excitability through suppression of sustained potassium channel currents of rat trigeminal ganglion neurons. Mol Pain. 2013;9:4. doi: 10.1186/1744-8069-9-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Okubo K, Matsumura M, Kawaishi Y, et al. Hydrogen sulfide-induced mechanical hyperalgesia and allodynia require activation of both Cav3.2 and TRPA1 channels in mice. Br J Pharmacol. 2012;166:1738–43. doi: 10.1111/j.1476-5381.2012.01886.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Distrutti E, Cipriani S, Renga B, et al. Hydrogen sulphide induces micro opioid receptor-dependent analgesia in a rodent model of visceral pain. Mol Pain. 2010;6:36. doi: 10.1186/1744-8069-6-36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Miyamoto T, Dubin AE, Petrus MJ, Patapoutian A. TRPV1 and TRPA1 mediate peripheral nitric oxide-induced nociception in mice. PLoS One. 2009;4:e7596. doi: 10.1371/journal.pone.0007596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Hervera A, Negrete R, Leánez S, et al. Peripheral effects of morphine and expression of μ-opioid receptors in the dorsal root ganglia during neuropathic pain: nitric oxide signaling. Mol Pain. 2011;7:25. doi: 10.1186/1744-8069-7-25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Freire MA, Guimarães JS, Leal WG, Pereira A. Pain modulation by nitric oxide in the spinal cord. Front Neurosci. 2009;3:175–81. doi: 10.3389/neuro.01.024.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Bijjem KR, Padi SS, lal Sharma P. Pharmacological activation of heme oxygenase (HO)-1/carbon monoxide pathway prevents the development of peripheral neuropathic pain in Wistar rats. Naunyn Schmiedebergs Arch Pharmacol. 2013;386:79–90. doi: 10.1007/s00210-012-0816-1. [DOI] [PubMed] [Google Scholar]
- 63.Nascimento CG, Branco LG. Antinociception synergy between the peripheral and spinal sites of the heme oxygenase-carbon monoxide pathway. Braz J Med Biol Res. 2009;42:141–47. doi: 10.1590/s0100-879x2009000100020. [DOI] [PubMed] [Google Scholar]
- 64.Hervera A, Gou G, Leánez S, Pol O. Effects of treatment with a carbon monoxide-releasing molecule and a heme oxygenase 1 inducer in the antinociceptive effects of morphine in different models of acute and chronic pain in mice. Psychopharmacology (Berl) 2013;228:463–77. doi: 10.1007/s00213-013-3053-5. [DOI] [PubMed] [Google Scholar]
- 65.Andrade P, Visser-Vandewalle V, Hoffmann C, et al. Role of TNF-alpha during central sensitization in preclinical studies. Neurol Sci. 2011;32:757–71. doi: 10.1007/s10072-011-0599-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Park CK, Lü N, Xu ZZ, et al. Resolving TRPV1- and TNF-α-mediated spinal cord synaptic plasticity and inflammatory pain with neuroprotectin D1. J Neurosci. 2011;31:15072–85. doi: 10.1523/JNEUROSCI.2443-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Wang F, Xu S, Shen X, et al. Spinal macrophage migration inhibitory factor is a major contributor to rodent neuropathic pain-like hypersensitivity. Anesthesiology. 2011;114:643–59. doi: 10.1097/ALN.0b013e31820a4bf3. [DOI] [PubMed] [Google Scholar]
- 68.Alexander JK, Cox GM, Tian JB, et al. Macrophage migration inhibitory factor (MIF) is essential for inflammatory and neuropathic pain and enhances pain in response to stress. Exp Neurol. 2012;236:351–62. doi: 10.1016/j.expneurol.2012.04.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Sun Y, Wang Y, Li JH, et al. Macrophage migration inhibitory factor counter-regulates dexamethasone-induced Annexin 1 expression and influences the release of eicosanoids in murine macrophages. Immunology. 2013;140:250–58. doi: 10.1111/imm.12135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Koh IJ, Chang CB, Lee JH, et al. Preemptive Low-dose Dexamethasone Reduces Postoperative Emesis and Pain After TKA: A Randomized Controlled Study. Clin Orthop Relat Res. 2013;471:3010–20. doi: 10.1007/s11999-013-3032-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.de Oliveira CM, Sakata RK, Issy AM, et al. Cytokines and pain. Rev Bras Anestesiol. 2011;61:260–65. doi: 10.1016/S0034-7094(11)70029-0. [DOI] [PubMed] [Google Scholar]
- 72.Ren K, Torres R. Role of interleukin-1beta during pain and inflammation. Brain Res Rev. 2009;60:57–64. doi: 10.1016/j.brainresrev.2008.12.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Guo W, Wang H, Watanabe M, et al. Glial-cytokine-neuronal interactions underlying the mechanisms of persistent pain. J Neurosci. 2007;27:6006–18. doi: 10.1523/JNEUROSCI.0176-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Leibinger M, Müller A, Gobrecht P, et al. Interleukin-6 contributes to CNS axon regeneration upon inflammatory stimulation. Cell Death Dis. 2013;4:e609. doi: 10.1038/cddis.2013.126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Muja N, DeVries GH. Prostaglandin E(2) and 6-keto-prostaglandin F(1alpha) production is elevated following traumatic injury to sciatic nerve. Glia. 2004;46:116–29. doi: 10.1002/glia.10349. [DOI] [PubMed] [Google Scholar]
- 76.Kunori S, Matsumura S, Okuda-Ashitaka E, et al. A novel role of prostaglandin E2 in neuropathic pain: blockade of microglial migration in the spinal cord. Glia. 2011;59:208–18. doi: 10.1002/glia.21090. [DOI] [PubMed] [Google Scholar]
- 77.Okuda-Ashitaka E, Minami T, Matsumura S, et al. The opioid peptide nociceptin/orphanin FQ mediates prostaglandin E2-induced allodynia, tactile pain associated with nerve injury. Eur J Neurosci. 2006;23:995–1004. doi: 10.1111/j.1460-9568.2006.04623.x. [DOI] [PubMed] [Google Scholar]
- 78.Shen X, Yuan H, Wang W, et al. Homeostatic synaptic plasticity: balanced by COX2-PGE2 system to a new setpoint. Sci Insights. 2013;1:20–23. [Google Scholar]
- 79.Dray A, Perkins M. Bradykinin and inflammatory pain. Trends Neurosci. 1993;16:99–104. doi: 10.1016/0166-2236(93)90133-7. [DOI] [PubMed] [Google Scholar]
- 80.Banik RK, Kozaki Y, Sato J, et al. B2 receptor-mediated enhanced bradykinin sensitivity of rat cutaneous C-fiber nociceptors during persistent inflammation. J Neurophysiol. 2001;86:2727–35. doi: 10.1152/jn.2001.86.6.2727. [DOI] [PubMed] [Google Scholar]
- 81.Nakamura Y, Izumi H, Shimizu T, et al. Volume transmission of substance P in striatum induced by intraplantar formalin injection attenuates nociceptive responses via activation of the neurokinin 1 receptor. J Pharmacol Sci. 2013;121:257–71. doi: 10.1254/jphs.12218fp. [DOI] [PubMed] [Google Scholar]
- 82.López-Bellido R, Barreto-Valer K, Rodríguez RE. Substance P mRNA expression during zebrafish development: influence of mu opioid receptor and cocaine. Neuroscience. 2013;242:53–68. doi: 10.1016/j.neuroscience.2013.03.022. [DOI] [PubMed] [Google Scholar]
- 83.Nazarian A, Tenayuca JM, Almasarweh F, et al. Sex differences in formalin-evoked primary afferent release of substance P. Eur J Pain. 2014;18:39–46. doi: 10.1002/j.1532-2149.2013.00346.x. [DOI] [PubMed] [Google Scholar]
- 84.Wei T, Guo TZ, Li WW, et al. Keratinocyte expression of inflammatory mediators plays a crucial role in substance P-induced acute and chronic pain. J Neuroinflammation. 2012;9:181. doi: 10.1186/1742-2094-9-181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Wei T, Li WW, Guo TZ, et al. Post-junctional facilitation of Substance P signaling in a tibia fracture rat model of complex regional pain syndrome type I. Pain. 2009;144:278–86. doi: 10.1016/j.pain.2009.04.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Kingery WS, Davies MF, Clark JD. A substance P receptor (NK1) antagonist can reverse vascular and nociceptive abnormalities in a rat model of complex regional pain syndrome type II. Pain. 2003;104:75–84. doi: 10.1016/s0304-3959(02)00467-0. [DOI] [PubMed] [Google Scholar]
- 87.Kashiba H, Fukui H, Morikawa Y, Senba E. Gene expression of histamine H1 receptor in guinea pig primary sensory neurons: a relationship between H1 receptor mRNA-expressing neurons and peptidergic neurons. Brain Res Mol Brain Res. 1999;66:24–34. doi: 10.1016/s0169-328x(98)00346-5. [DOI] [PubMed] [Google Scholar]
- 88.Nuutinen S, Panula P. Histamine in neurotransmission and brain diseases. Adv Exp Med Biol. 2010;709:95–107. doi: 10.1007/978-1-4419-8056-4_10. [DOI] [PubMed] [Google Scholar]
- 89.Deval E, Gasull X, Noël J, et al. Acid-sensing ion channels (ASICs): pharmacology and implication in pain. Pharmacol Ther. 2010;128:549–58. doi: 10.1016/j.pharmthera.2010.08.006. [DOI] [PubMed] [Google Scholar]
- 90.Holzer P. Acid sensing by visceral afferent neurones. Acta Physiol (Oxf) 2011;201:63–75. doi: 10.1111/j.1748-1716.2010.02143.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Hayashi K, Shiozawa S, Ozaki N, et al. Repeated intramuscular injections of nerve growth factor induced progressive muscle hyperalgesia, facilitated temporal summation, and expanded pain areas. Pain. 2013;154:2344–52. doi: 10.1016/j.pain.2013.07.007. [DOI] [PubMed] [Google Scholar]
- 92.Osikowicz M, Longo G, Allard S, et al. Inhibition of endogenous NGF degradation induces mechanical allodynia and thermal hyperalgesia in rats. Mol Pain. 2013;9:37. doi: 10.1186/1744-8069-9-37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Shaqura M, Khalefa BI, Shakibaei M, et al. Reduced number, G protein coupling, and antinociceptive efficacy of spinal mu-opioid receptors in diabetic rats are reversed by nerve growth factor. J Pain. 2013;14:720–30. doi: 10.1016/j.jpain.2013.01.776. [DOI] [PubMed] [Google Scholar]
- 94.Webber CA, Salame J, Luu GL, et al. Nerve growth factor acts through the TrkA receptor to protect sensory neurons from the damaging effects of the HIV-1 viral protein, Vpr. Neuroscience. 2013;252:512–25. doi: 10.1016/j.neuroscience.2013.07.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Kobayashi K, Yamanaka H, Yanamoto F, et al. Multiple P2Y subtypes in spinal microglia are involved in neuropathic pain after peripheral nerve injury. Glia. 2012;60:1529–39. doi: 10.1002/glia.22373. [DOI] [PubMed] [Google Scholar]
- 96.Mo G, Grant R, O’Donnell D, et al. Neuropathic Nav1.3-mediated sensitization to P2X activation is regulated by protein kinase C. Mol Pain. 2011;7:14. doi: 10.1186/1744-8069-7-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Kanno T, Yaguchi T, Nishizaki T. Noradrenaline stimulates ATP release from DRG neurons by targeting beta(3) adrenoceptors as a factor of neuropathic pain. J Cell Physiol. 2010;224:345–51. doi: 10.1002/jcp.22114. [DOI] [PubMed] [Google Scholar]
- 98.Burnstock G, Krügel U, Abbracchio MP, Illes P. Purinergic signalling: from normal behaviour to pathological brain function. Prog Neurobiol. 2011;95:229–74. doi: 10.1016/j.pneurobio.2011.08.006. [DOI] [PubMed] [Google Scholar]