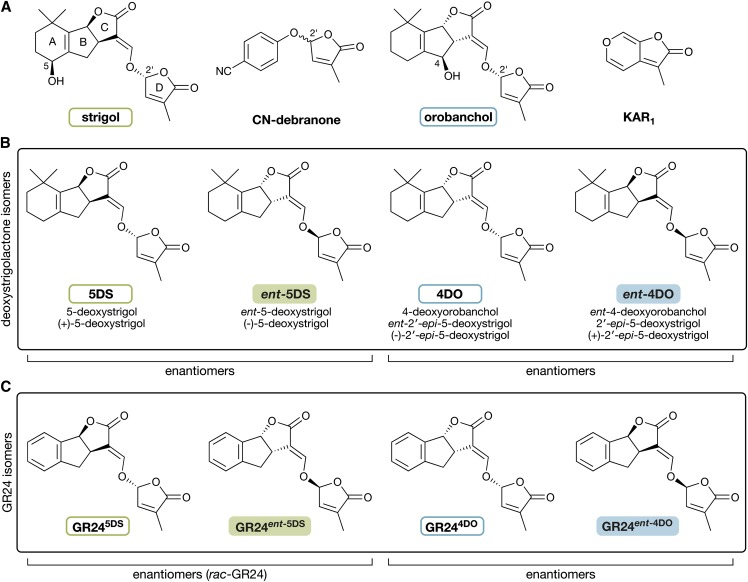
Figure 1.
Chemical structures of SL stereoisomers and related butenolide compounds. A, Two naturally occurring SLs, strigol and orobanchol, with the characteristic ABC-ring to D-ring structure. Note the hydroxyl groups at the C5 and C4 positions and the differing stereochemistry at the B-ring to C-ring junctions. The stereochemistry at C2′ is the same in both strigol and orobanchol. KAR1 is an achiral seed germination stimulant found in burnt plant material. CN-debranone is a compound with SL-like activity. Note the single chiral center at the C2′ position; both stereoisomers are depicted in the single structure. B, The deoxySLs 5DS and 4DO and their corresponding enantiomers (mirror images). Below each compound is a list of possible synonyms. The enantiomers ent-5DS and ent-4DO have not been reported to be produced in nature. C, The GR24 group of artificial SL analogs. Each is an analog of a corresponding deoxySL, which is denoted by the superscript suffix.