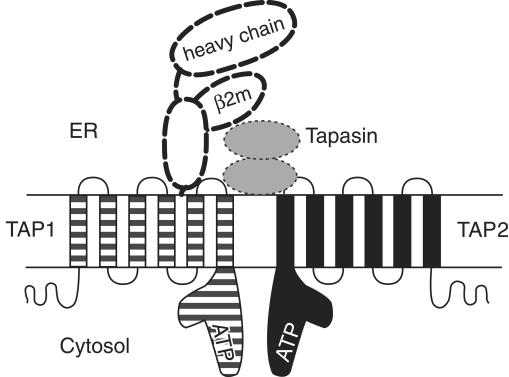
Figure 1.
Selected components of the MHC class I antigen presentation pathway. The structures of individual components, modes of interaction between the different components, and stoichiometries of binding remain to be fully defined. TAP1 (solid line, striped) and TAP2 (solid line, black) each contain multiple membrane-spanning segments and C–terminal ATP-binding domains, depicted here as L-shaped structures, based upon the crystal structure of analogous domains of a bacterial histidine transporter (15). TAP1 and TAP2 interact to form a functional peptide transporting complex. In the ER lumen, translocated peptides associate with MHC class I heavy chains and β2m (bold dashed lines) (16). Peptide binding by MHC class I molecules is facilitated by the transient formation of TAP–MHC class I complexes. This interaction involves tapasin (dotted lines, gray) as well as calreticulin (2), a cellular chaperone (not shown in the figure). Tapasin interactions with MHC class I molecules may resemble class I interactions with CD8, a coreceptor for the T-cell receptor (17). Defects in either TAP1 or TAP2 result in defective assembly of class I heterodimers and have been shown to cause human MHC class I deficiencies (7, 9, 10). It is likely that other cases of MHC class I deficiencies will map to mutations that interfere with the stable expression of additional components of the MHC class I antigen presentation pathway, or with interactions between the different components.