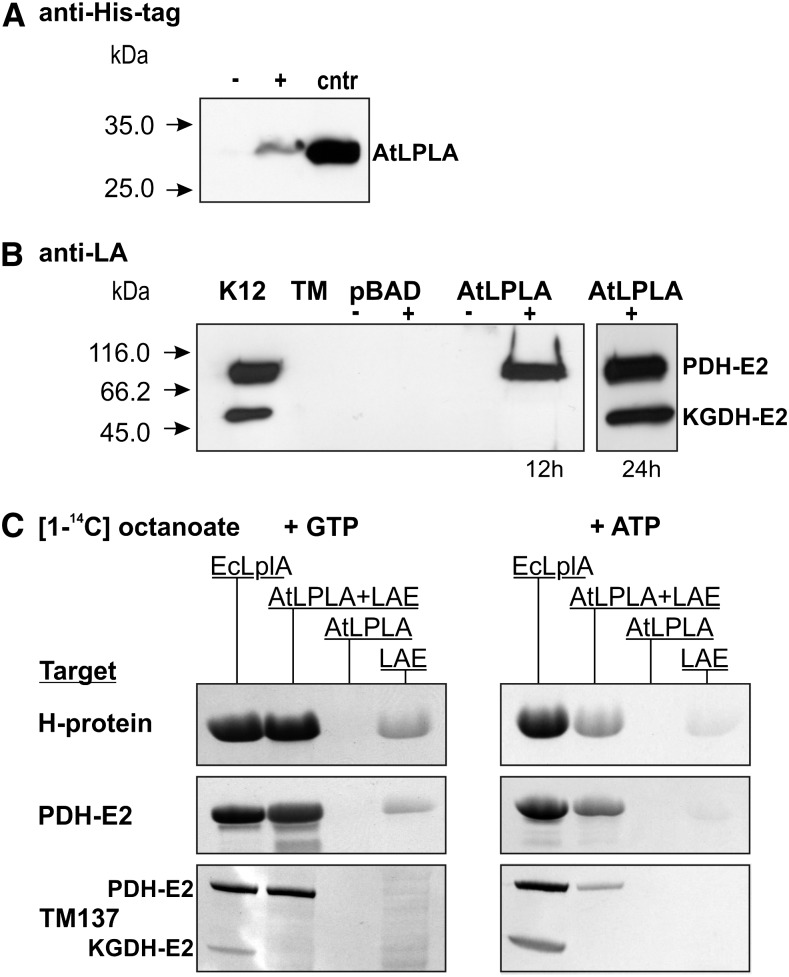
Figure 2.
Activity of AtLPLA in vivo and in vitro. A, AtLPLA is expressed in TM137, as shown with a His tag-specific antibody. Ten micrograms of total soluble protein was loaded per lane from uninduced (−) and induced (+) TM137 pBAD-HisA-LPLA cells. Five micrograms of affinity-purified His-tagged AtLPLA was loaded as a control (cntr). B, AtLPLA expression in TM137 results in rapid lipoylation of E. coli PDH-E2 and very slow lipoylation of KGDH-E2. K12, E. coli K12 control; TM, LplA− LipB− strain TM137; pBAD, empty pBAD-HisA vector transformed into TM137; AtLPLA, pBAD-HisA-LPLA transformed into TM137 after 12 and 24 h of growth without (−) and with (+) induction of expression with 0.2% Ara and appropriate antibiotics. C, AtLPLA requires substrate activation by a separate enzyme. The autoradiograms show [14C]octanoyl signals after SDS-PAGE of in vitro assay mixtures with 0.19 mm [1-14C]octanoic acid and 5 mm GTP (left) or ATP (right) and the three different target proteins Arabidopsis H-protein (5 µg), Arabidopsis PDH-E2 (2.5 µg), and TM137 soluble proteins (50 µg). Octanoate is not attached without NTP. Assay variants were 2.5 µg of E. coli LPLA as a positive control, 2.5 µg of AtLPLA plus 0.5 µg of bovine LAE, 2.5 µg of AtLPLA without LAE, and 0.5 µg of bovine LAE.