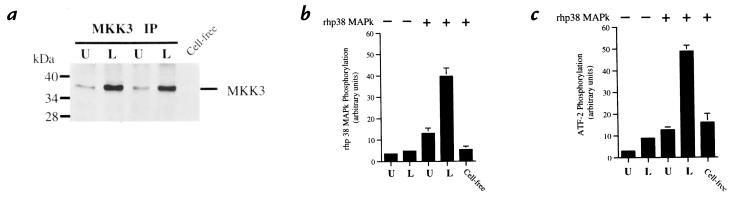
Figure 1.
Coupled assay of MKK3 activation. Neutrophils were stimulated with LPS (100 ng/ml) for 20 min at 37°C. (a) Phosphorylation of MKK3. MKK3 was immunoprecipitated from cell lysates of LPS-stimulated (L) or unstimulated (U) neutrophils. Lysates were submitted to SDS-PAGE and Western blotting with an anti-phosphorylated MKK3 antibody. A cell-free mixture that did not contain immunoprecipitated MKK3 is shown to control for intrinsic activity of the rhp38 MAPk. Blot is representative of three experiments. (b) Activation of MKK3. 32P phosphorylation of rhp38 MAPk from blots was quantified by phosphor screen autoradiography. Lysates in the absence of rhp38 MAPk (lanes 1 and 2) were compared with lysates in the presence of rhp38 MAPk (lanes 3 and 4) and a cell-free control to demonstrate specific activation of MKK3 in response to LPS as determined by 32P phosphorylation of rhp38 MAPk. (c) Coupled activation of rhp38 MAPk by activated MKK3. The ability of activated MKK3 to activate rhp38 MAPk was determined by the ability of phosphorylated rhp38 MAPk to phosphorylate the substrate ATF-21-110. 32P phosphorylation of ATF-21-110 from blots was quantified by phosphor screen autoradiography. Lysates in the absence of rhp38 MAPk (lanes 1 and 2) represent baseline phosphorylation of the ATF-21-110, whereas lysates in the presence of rhp38 MAPk (lanes 3 and 4) demonstrate increased phosphorylation of ATF-21-110 via coupled activation of rhp38 MAPk by activated MKK3 in response to LPS. The cell-free control quantifies intrinsic activity of the rhp38 MAPk and is equivalent to the unstimulated lysate in lane 3. Plots depict mean values and SEM from three consecutive experiments expressed in arbitrary units. ATF, activated transcription factor; LPS, lipopolysaccharide; MAPk, mitogen-activated protein kinase; MKK, MAPk kinase.