Abstract
Methanandamide acts at targets which modulate amphetamine-induced behaviors. Therefore, we investigated methanandamide effects on the acute hyperactivity produced by a single injection of amphetamine and behavioral sensitization induced by repeated amphetamine exposure in rats. Methanandamide (5 mg/kg, i.p.) did not affect basal locomotor or stereotypical activity. Methanandamide (5 mg/kg, i.p.) pretreatment did not alter the acute increase in locomotor or stereotypical activities produced by acute amphetamine (2 mg/kg, i.p.). For chronic studies, rats injected with amphetamine (2 mg/kg, i.p.) once daily for 3 consecutive days were then challenged with amphetamine (2 mg/kg, i.p.) 5 days later. Expression of locomotor sensitization was blocked when methanandamide (5 mg/kg, i.p.) was given once, just prior to amphetamine (2 mg/kg, i.p.) challenge. In rats co-exposed to methanandamide (5 mg/kg, i.p.) and amphetamine (2 mg/kg, i.p.) on days 1-3 and then challenged with amphetamine (2 mg/kg, i.p.) following 5 days of drug absence, the development of both locomotor and stereotypical sensitization was blocked. The ability of methanandamide to block amphetamine-sensitized behaviors suggests that this pharmacologically diverse lipid regulates signaling events impacted by repeated psychostimulant exposure.
Keywords: metahanandamide, anandamide, amphetamine, sensitization, psychostimulant, locomotor, stereotypy, vanilloid, cannabinoid
1. Introduction
Chronic amphetamine exposure produces behavioral sensitization in rats and mice (Robinson and Camp, 1987; Kalivas and Stewart, 1991; Paulson and Robinson, 1995). In this process, animals exposed initially to amphetamine display behavioral activation manifested as an increase in locomotor activity and stereotypy (Creese and Iversen, 1975). When the same animal is exposed repeatedly to amphetamine, undergoes a period of abstinence, and is then reintroduced to the drug, it results in behavioral activation of a greater magnitude than that seen with initial amphetamine exposure. Because behavioral sensitization is enduring, neural adaptations which underlie sensitization have been hypothesized to mediate relapse in drug dependence (Paulson et al., 1991; Kalivas and Stewart, 1991; Robinson and Berridge, 1993; Vezina, 2004). Neurochemical sensitization, a process in which repeated drug exposure results in exaggerated dopaminergic and glutamatergic transmission in the striatum, can also be elicited by amphetamine (Kalivas and Stewart, 1991; Robinson and Becker, 1982; Robinson and Berridge, 1993; Robinson et al., 1988; Vezina, 2004).
Although key roles for dopamine and glutamate in amphetamine-induced behavioral sensitization have been established using numerous approaches (Vanderschuren and Kalivas, 2000), it is probable that additional neurotransmitters, receptors and ion channels modulate a process as complex as sensitization. In cases in which a process is mediated by more than one mechanism, a useful approach for blocking the process is to use a diverse agent with the capacity to induce biological effects by acting at multiple sites of action. One drug which displays this property is methanandamide, a stable analog of the brain lipid anandamide. Methanandamide is more stable than anandamide because it displays increased resistance to fatty acid amide hydrolase (FAAH) metabolism compared to anandamide (Pertwee, 1997, Mechoulam et al., 1998; Muschamp et al., 2002; Smith et al., 2007; Romero et al., 1995, 1996). Prior work underscores the diversity of methanandamide as the agent is known to activate transient receptor potential vanilloid 1 cation (TRPV1) channels, inhibit TASK-1 K+ channels, inhibit transient (T)-type Ca2+ channels, activate cannabinoid CB1 and CB2 receptors, facilitates electrogenic glycine transport, enhances glycine receptor function and allosterically inhibit nicotinic acetylcholine receptors (Maingret et al., 2001; Oz, 2006; Zygmunt et al., 1999; Malinowska et al., 2001; Chemin et al., 2001; Hejazi et al., 2006; Di Marzo et al., 2001; Fride and Mechoulam, 1993; Devane et al., 1992; Pearlman et al., 2003; Baranowska et al., 2008). Because several of these targets modulate psychostimulant-induced behaviors, we investigated the effect of methanandamide on acute hyperactivity produced by a single injection of amphetamine and behavioral sensitization caused by repeated amphetamine exposure.
2. Methods
2.1. Animals
Adult male Sprague-Dawley rats 275-350 g were purchased from Ace Animal (Boyer Town, PA, USA) housed 2-3 per cage and maintained on a 12-hour light-dark cycle and given rat chow and water ad libitum. All animal use procedures were conducted in strict accordance with the NIH Guide for the Care and Use of Laboratory Animals and were approved by the Temple University Institutional Animal Care and Use Committee.
2.2. Drugs
(R)-(+)-methanandamide was purchased from Tocris Bioscience (Ellisville, MI, USA), came dissolved in TocrisolveTM and diluted in normal saline to a concentration of 2.5 mg/ml. d-amphetamine was provided by the National Institutes on Drug Abuse. Drugs were administered in a volume of 1 ml/kg.
2.3. Behavioral experiments
Activity was detected with the Digiscan Dmicro System and analyzed with Micropro version 1.3 software (Accuscan Instruments, Columbus, OH, USA) as previously described (Soderman and Unterwald, 2008). Eight adjacent Plexiglas chambers (similar to home cages without bedding) were placed in frames which were each equipped with 16 infrared beams. Activity was recorded and tallied in 10-min bins for 100 min following the i.p. injection of amphetamine or saline. Locomotor behavior was registered when consecutive light beams were interrupted. Stereotypy was detected when the same light beam was repeatedly broken. Light beam height was raised ∼1 cm in experiment 3. This change may explain the reduction in score magnitudes relative to experiments 1 and 2. The rats were acclimated for 1 hr in the test room prior and tested in the AM of the light cycle.
2.4. Dosing paradigms
Three separate experiments examined the effects of methanandamide on amphetamine-induced hyperactivity.
Experiment 1.Effect of methanandamide on acute hyperactivity induced by amphetamine
Rats were pretreated with either methanandamide (5 mg/kg i.p.) or equivalent volume vehicle and immediately placed in test chambers. Twenty min later amphetamine (2 mg/kg i.p.) or saline was injected.
Experiment 2. Effect of methanandamide on expression of amphetamine-induced behavioral sensitization
Rats co-treated with vehicle and amphetamine in Experiment 1 were injected again with vehicle and amphetamine at the same doses on days 2 and 3 in their home cages. This group was subsequently split and challenged with amphetamine on day 8 after having been pretreated 20 min earlier with methanandamide (5 mg/kg i.p.) or vehicle. This procedure was repeated with lower doses of methanandamide (1.25 or 2.5 mg/kg i.p.).
Experiment 3. Effect of methanandamide on development of amphetamine-induced behavioral sensitization
On days 1-3, rats were administered methanandamide (5 mg/kg i.p.) or vehicle and injected 20 min later with saline or amphetamine (2 mg/kg) in their home cages. On day 8 all rats were challenged with amphetamine (2 mg/kg i.p.).
2.5. Statistics
Data were presented as total mean activity counts in 10-min bins. Time-course data were analyzed by one-way repeated measures ANOVA. In cases in which a significant main effect was present, a Bonferroni's post-hoc analysis was used to compare individual groups. P < 0.05 was considered statistically significant.
3. Results
3.1. Effect of methanandamide on acute amphetamine-induced hyperactivity
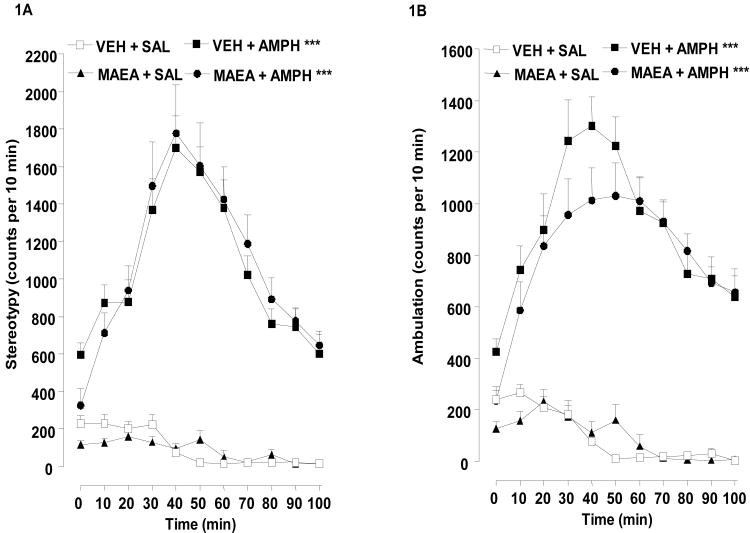
Fig. 1A and 1B show the effects of methanandamide pretreatment on acute hyperactivity produced by amphetamine. There was a significant main effect for stereotypy count, F(3,21) = 65.04, P < 0.0001 (Fig. 1A). Post-hoc analysis confirmed the stimulatory effect of amphetamine with the vehicle plus amphetamine and methanandamide plus amphetamine groups increased over the methanandamide plus saline and vehicle plus saline control groups. Conversely, the amphetamine-treated groups were not different from each other while the control groups also did not differ from each other. Results for a main effect of amphetamine locomotor activity were similar, F(3,21) = 99.87, P < 0.0001 (Fig. 1B). Post-hoc comparisons were also identical in significance to those for stereotypy count, P < 0.001 in all cases, thus indicating that methanandamide, administered in this dosing regimen, does not significantly alter acute hyperactivity induced by amphetamine.
Fig. 1.
Effect of methanandamide on acute hyperactivity induced by a single injection of amphetamine. Animals received methanandamide (MAEA) (5 mg/kg) or vehicle (VEH) 20 min before amphetamine (AMPH) (2 mg/kg, i.p.) or saline (SAL), and stereotypy (a) and locomotor (b) counts were measured 100 min post-injection. Data from 7-8 rats per group are expressed as mean (S.E.M.) stereotypy or locomotor counts per 10 min intervals. Time course ANOVA: ***P < 0.001 compared to VEH + SAL group.
3.2. Effect of methanandamide on expression of amphetamine-induced behavioral sensitization
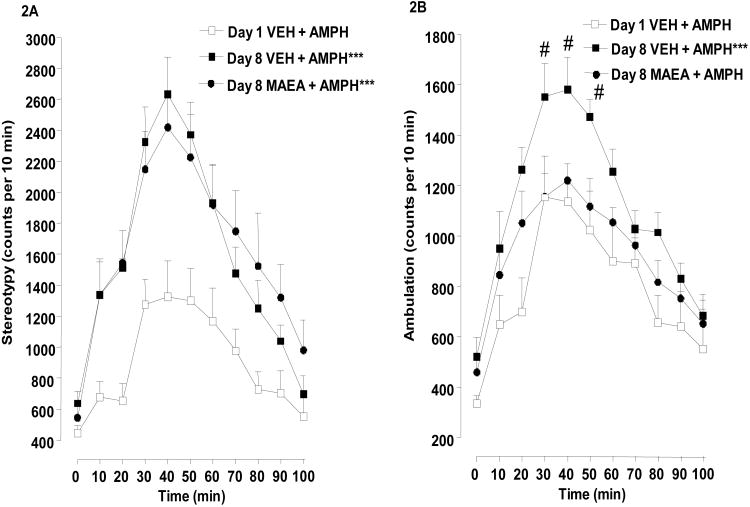
Effects of methanandamide (5 mg/kg i.p.) on the expression of behavioral sensitization caused by amphetamine administration are shown in Fig. 2. Fig. 2A displays the results for stereotypy count. Repeated-measures ANOVA on the data found a significant main effect for increase in stereotypy from day 1 to day 8, F(2,14) = 84.17, P < 0.0001. Post-hoc analysis showed that both the vehicle- and methanandamide-pretreated groups were significantly (P < 0.001) increased in stereotypy on day 8 in comparison to day 1. Further analysis revealed that day 8 amphetamine challenge did not produce stereotypy that was significantly different in rats pretreated with vehicle or 5 mg/kg of methanandamide (P > 0.05). Pretreatment with lower methanandamide doses (1.25 or 2.5 mg/kg) also failed to alter the stereotypy produced by day 8 amphetamine challenge (i.e., 0.96 ± 0.10% for 1.25 mg/kg methanandamide and 0.96 ± 0.09% for 2.5 mg/kg methanandmide, expressed as the percentage of total stereotypy counts produced by day 8 amphetamine administration in the vehicle-pretreated group) (P > 0.05).
Fig. 2.
Effect of methanandamide on expression of amphetamine-induced behavioral sensitization. Animals received injections of vehicle (VEH) plus amphetamine (AMPH) (2 mg/kg, i.p.) for 3 days. Five days after the last AMPH administration, rats injected with VEH or methanandamide (MAEA) (2 mg/kg, i.p.) were administered a challenge dose of AMPH (2 mg/kg, i.p.) 20 min later. Stereotypy (a) and locomotor (b) counts were measured for 100 min post-injection. Data from 7-8 rats per group are expressed as mean (S.E.M.) stereotypy or locomotor counts per 10 min intervals. Time course ANOVA: *P < 0.05 or ***P < 0.001 compared to Day 1 VEH + AMPH and +P < 0.05 compared to Day 8 VEH + AMPH. Time point comparisons: #P < 0.05 compared to Day 8 MAEA + AMPH.
Corresponding data for locomotor activity are presented in Fig. 2B. Repeated-measures ANOVA revealed a significant main effect F(2,14) = 41.57, P < 0.0001. Post-hoc analysis confirmed the increase in activity from day 1 to day 8 for the vehicle plus amphetamine group (P < 0.001). In contrast to results for stereotypy, however, amphetamine administration on day 8 produced significantly less locomotor activity in rats pretreated with 5 mg/kg of methanandamide than in rats pretreated with vehicle (P < 0.001). Pretreatment with lower methanandamide doses (1.25 or 2.5 mg/kg) did not significantly reduce locomotor activity produced by day 8 amphetamine challenge (i.e., 0.89 ± 0.07% for 1.25 mg/kg methanandamide and 0.99 +/-0.08 % for 2.5 mg/kg methanandmide, expressed as the percentage of total locomotor counts produced by day 8 amphetamine administration in the vehicle-pretreated group was) (P > 0.05). Post-hoc analysis also revealed a difference between the day 1 vehicle plus amphetamine and the day 8 methanandamide plus amphetamine groups (P < 0.05). This effect may be an artifact of the irregularly shaped curve for the day 1 vehicle plus amphetamine group. These data indicate that methanandamide attenuated the expression of increased locomotor, but not stereotypic activity resultant from previous amphetamine treatment.
3.3. Effect of methanandamide on development of amphetamine-induced behavioral sensitization
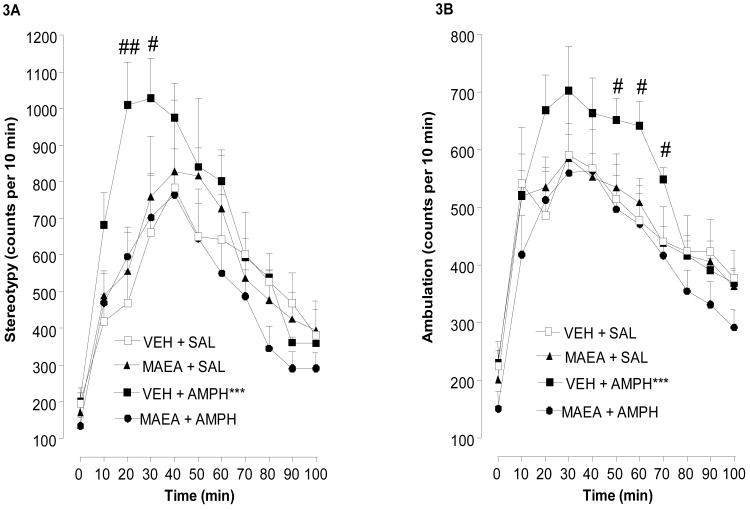
Fig. 3A presents the effect of methanandamide on development of sensitized stereotypy. Repeated-measures ANOVA on the data revealed a significant main effect on stereotypy F(3,21) = 13.17, P < 0.0001]. Post-hoc analysis indicated that rats treated only with amphetamine during the conditioning phase were significantly increased in stereotypy count compared to all of the other groups when challenged with amphetamine (VEH + AMPH vs. MAEA + AMPH, p < 0.001; VEH + AMPH vs. MAEA + SAL, p < 0.01; VEH + AMPH vs. VEH + SAL, P < 0.001. None of the other groups were significantly different from each other. These data indicate the methanandamide pretreatment during the conditioning phase blocked amphetamine-induced sensitization in stereotypy count. The data also indicated that repeated methanandamide treatment alone during the conditioning phase did not alter the acute effect of amphetamine.
Fig. 3.
Effect of methanandamide on development of amphetamine-induced behavioral sensitization. On days 1-3, animals injected with vehicle (VEH) or methanandamide (MAEA) (2 mg/kg, i.p.) were injected 20 min later with (AMPH) (2 mg/kg, i.p.) or saline (SAL). Five days after the last AMPH administration, all rats were administered a challenge dose of AMPH (2 mg/kg, i.p.). Stereotypy (a) and locomotor (b) counts were measured 100 min post-injection. Data from 7-8 rats per group are expressed as the mean ± S.E.M. of stereotypy or locomotor counts per 10 min intervals. Time course ANOVA: ***P < 0.001 compared to VEH + SAL and +++P < 0.05 compared to VEH + AMPH. Time point comparisons: #P < 0.05 or ##P < 0.01 compared to MAEA + AMPH.
Corresponding data for the effects of methanandamide on the development of amphetamine-sensitized locomotor activity are shown in Fig. 3B. Over all, the data were very similar to those for stereotypy. Repeated-measures ANOVA demonstrated a significant main effect F(3,21) = 21.24, P < .0001. Post-hoc analysis revealed that, as was the case with stereotypy count, the vehicle plus amphetamine group was significantly increased over all of the other groups following the amphetamine challenge (P < 0.001 for all comparisons). As with the data for locomotor activity, there were no significant differences between the other groups.
4. Discussion
We demonstrated that methanandamide blocks the development and expression of amphetamine-induced behavioral sensitization. Methanandamide acts at multiple targets, including cannabinoid CB1 and CB2 receptors, TRPV1 channels, TASK-1 K+ channels, T-type Ca2+ channels, glycine receptors, and nicotinic acetylcholine receptors (Oz, 2006; Zygmunt et al., 1999; Malinowska et al., 2001; Chemin et al., 2001; Hejazi et al., 2006; Zygmunt et al., 1999; Di Marzo et al., 2001; Fride and Mechoulam, 1993; Devane et al., 1992; Baranowska et al., 2008). Although the cellular site of action responsible for the effects of methanandamide demonstrated here is unknown, one which is both activated by methanandamide and involved in amphetamine sensitization is likely. The nicotinic acetylcholine receptor is one possibility. Nicotinic receptor activation by endogenously released acetylcholine contributes to the development of amphetamine-induced behavioral sensitization in rats (Schoffelmeer et al., 2002; de Rover et al., 2004). Evidence that methanandamide noncompetitively inhibits nicotinic receptors in vivo and in vitro suggests that a nicotinic receptor block in rats co-exposed to methanandamide and amphetamine may have disrupted processes underlying the development of behavioral sensitization (Baranowska et al., 2008; Oz et al., 2004). Another possibility is that methanandamide acted at ion channels (e.g. TASK-1, T-type, voltage-sensitive Na+ channels, TRPV1) which shape synaptic activity (Maingret et al., 2001; Chemin et al., 2001; Zygmunt et al., 1999; Nicholson et al., 2003; Roberts et al., 2008). Changes in ion channel activities during repeated psychostimulant exposure do underlie synaptic modifications in the mesolimibic dopamine system, and associated limbic structures, that contribute to specific aspects of addiction (Christie et al., 1997; Su et al., 2002; Kauer and Malenka, 2007). Methanandamide, by acting at one or more of these channels, may have counteracted the normal synaptic modifications produced by repeated amphetamine exposure, resulting in an inhibition of amphetamine-sensitized behavioral responses. Methanandamide also enhances glycine receptor function through a cannabinoid and TRPV1 receptor-independent pathway (Lozovaya et al., 2005; Hezaji et al., 2006). Yet, it is unlikely that glycine receptors mediated the effects of methanandamide observed here because their activation does not affect sensitization of amphetamine-induced locomotor activity (Hezaji et al., 2006; Przegaliński et al., 1999).
Cannabinoid CB1 receptors are activated by methanandamide, but their role in psychostimulant-induced behavioral sensitization remains unclear (Di Marzo et al., 2001; Fride and Mechoulam, 1993; Devane et al., 1992). Although CB1 receptors are expressed by dopaminergic neurons that mediate the development and expression of amphetamine-induced behavioral sensitization, behavioral studies have yielded mixed results (Herkenham et al., 1991; Wenger et al., 2003; Arnold, 2005). For example, mice co-treated with SR 141716A, a CB1 receptor antagonist, and amphetamine display enhanced locomotor sensitization to amphetamine challenge whereas amphetamine-induced locomotor sensitization is reduced in mice lacking CB1 receptors or treated with the CB1 receptor antagonist AM 251 (Corbrille et al., 2007; Thiemann et al., 2008a, b). Additionally, SR141716A does not prevent development of cocaine-induced behavioral sensitization but blocks expression of the behavior (Lesscher et al., 2005; Filip et al., 2006). Those studies used pharmacological and genetic approaches to investigate a role for endogenous cannabinoids on psychostimulant sensitization whereas we administered methanandamide exogenously. Thus, if methanandamide activated CB1 receptors to inhibit sensitization of amphetamine hyperactivity, then cannabinoid agonists such as WIN 55212-2 and CP 55,940 would be expected to produce similar effects on psychostimulant sensitization. However, rats treated daily with WIN 55,212-2 display an enhanced response to amphetamine with rearing but not with locomotor movements whereas pre-exposure or concurrent exposure to CP 55,940 does not enhance sensitivity to the subsequent behavioral effects of cocaine (Muschamp and Siviy, 2002; Arnold et al., 1998). The observation that methanandamide produced effects on sensitization that were different from WIN 55212-2 and CP 55,940 might be due to: (i) methanandamide acting through a cannabinioid-independent pathway; (ii) amphetamine and cocaine producing sensitization through processes that are not entirely similar (Vanderschuren and Kalivas, 2000; Seiden et al., 1993); dissimilarities in species, age, sex and genotypes of animals; and (iv) dissimilarities in experimental design; and (v) the capacity of methanandamide to simultaneously activate multiple targets, which may exert opposing and/or congruent effects on psychostimulant-induced behaviors.
Two of the more interesting findings in the present study were the dissimilar modulation by methanandamide of the expression of amphetamine-induced locomotor and stereotypical sensitization and the ineffectiveness of low methanandamide doses against both sensitized responses. Methanandamide prevented expression of sensitized locomotor activity but did not affect sensitized stereotypical behavior. It is widely accepted that amphetamine-induced behavioral sensitization is a multifactorial phenomenon involving many brain circuits, and evidence suggests that stereotypic and locomotor activation are not entirely mediated by the same neural substrates (Narendran and Martinez, 2008; Creese and Iversen, 1975; Kelly et al., 1975). Thus, one explanation for why methanandamide displayed greater efficacy versus expression of locomotor sensitization is that a locus such as the nucleus accumbens, a primary mediator of locomotor activation, may be more sensitive to methanandamide than a substrate such as the dorsal striatum, which is more closely associated with stereotypical activation (Creese and Iversen, 1975; Kelly et al., 1975). Methanandamide doses less than 5 mg/kg did not affect expression of either locomotor or stereotypical sensitization induced by amphetamine. It is possible that the lower methanandamide doses (1.25 and 2.5 mg/kg) chosen for our study were just below the threshold dose required for efficacy against amphetamine-induced sensitization. A related possibility is that one or more aspects of the sensitization paradigm itself, including the dose of amphetamine, frequency of amphetamine administration, and length of amphetamine withdrawal (i.e., time between discontinuation of repeated amphetamine exposure and amphetamine challenge), resulted in sensitization that was so significant as to mask any effectiveness of the lower doses. In this case, the lower methanandamide doses may have displayed efficacy in a paradigm which resulted in less intense sensitization, such as one which used a lower amphetamine dose or shorter withdrawal interval (Vanderschuren and Kalivas, 2000). Although conjectural, a mechanistic-based possibility is that TRPV1 receptor activation, produced only by the higher methanandamide dose, was primarily responsible for methanandamide efficacy. Evidences supporting this explanation are that: (a) TRPV1 receptors are abundant in limbic and basal ganglia substrates which mediate the process of sensitization (Mezey et al., 2000); (b) TRPV1 agonists reduce locomotor activity in rodents (Lee et al., 2006); (c) methanandamide activates TRPV1 receptors, but only at concentrations higher than those required to activate cannabinoid CB1 receptors (Zygmunt et al., 1999). Therefore, in our experiments, effects of TRPV1 receptor activation may have only been detectable following administration of the higher methanandamide dose. Future studies will address the mechanism and site of action of methanandamide as it relates to amphetamine-induced sensitization.
Methanandamide did not affect hyperactivity induced by acute amphetamine exposure, a finding consistent with previous work from our laboratory showing that rats pretreated with methanandamide display normal hyperactivity following acute cocaine exposure (Rasmussen et al., 2009). The inability of methanandamide to alter amphetamine- and cocaine-induced hyperactivity is different from the effects of other cannabinoid agonists on acute hyperactivity produced by psychostimulants. For example, pretreatment with δ9-THC doubles stereotyped gnawing in rats but does not affect hyperlocomotion produced by amphetamine (Moss et al., 1984). Additionally, cocaine-induced hyperlocomotion is counteracted by the synthetic cannabinoind agonist HU-210, but only at doses which suppress basal locomotor activity and stereotypy (Ferrari et al., 1999). The ineffectiveness of methanandamide in our experiments may be due to the dose used because 5 mg/kg does not affect basal locomotor activity or stereotypy (Rasmussen et al., 2009). Higher doses of methanandamide do reduce basal activity (Jarbe et al., 2003), and these doses might be capable of counteracting cocaine-induced hyperactivity in a manner similar to δ9-THC and WIN 55212-2. This explanation, however, does not account for the dissimilar effects of methanandamide and WIN 55212-2, which reduces cocaine-induced hyperactivity at doses that do not affect basal activity and by a mechanism that is not antagonized by SR 141716A (Przegaliński et al., 2005). Differences in efficacy and selectivity between methanandamide and WIN 55212-2 may account for the different results. WIN 55212-2 is a full agonist that produces maximal G-protein activation whereas methanandamide is unable to produce maximal activation of G-proteins, a phenomenon observed both at the level of G-protein activation and at downstream targets such as adenylyl cyclase and ion channels (Childers et al., 1994; Felder et al., 1993).
In conclusion, rats co-treated with amphetamine and methanandamide for 3 days and then challenged with amphetamine 5 days after the last injection did not display sensitized locomotor or stereotypical hyperactivity. More research is needed to understand the functional implications of these findings, but they do provide evidence that methanandamide regulates pathways and signaling events impacted by repeated amphetamine exposure.
Acknowledgments
This study was funded by National Institutes on Drug Abuse grants DA022694, DA025314, T32 DA07237-17 and P30-DA13429.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Arnold JC. The role of endocannabinoid transmission in cocaine addiction. Pharmacol Biochem Behav. 2005;81:396–406. doi: 10.1016/j.pbb.2005.02.015. [DOI] [PubMed] [Google Scholar]
- Arnold JC, Topple AN, Hunt GE, McGregor IS. Effects of pre-exposure and co-administration of the cannabinoid receptor agonist CP 55,940 on behavioral sensitization to cocaine. Eur J Pharmacol. 1998;354:9–16. doi: 10.1016/s0014-2999(98)00433-6. [DOI] [PubMed] [Google Scholar]
- Baranowska U, Göthert M, Rudz R, Malinowska B. Methanandamide allosterically inhibits in vivo the function of peripheral nicotinic acetylcholine receptors containing the alpha 7-subunit. J Pharmacol Exp Ther. 2008;326:912–919. doi: 10.1124/jpet.108.140863. [DOI] [PubMed] [Google Scholar]
- Chemin J, Monteil A, Perez-Reyes E, Nargeot J, Lory P. Direct inhibition of T-type calcium channels by the endogenous cannabinoid anandamide. EMBO J. 2001;20:7033–7040. doi: 10.1093/emboj/20.24.7033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Childers SR. Activation of G-proteins in brain by endogenous and exogenous cannabinoids. AAPS J. 2006;8:E112–117. doi: 10.1208/aapsj080113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Childers SR, Pacheco MA, Bennett BA, Edwards TA, Hampson RE, Mu J, Deadwyler SA. Cannabinoid receptors: G-protein-mediated signal transduction mechanisms. Biochem Soc Symp. 1993;59:27–50. [PubMed] [Google Scholar]
- Corbillé AG, Valjent E, Marsicano G, Ledent C, Lutz B, Hervé D, Girault JA. Role of cannabinoid type 1 receptors in locomotor activity and striatal signaling in response to psychostimulants. J Neurosci. 2007;27:6937–6947. doi: 10.1523/JNEUROSCI.3936-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Creese I, Iversen SD. The pharmacological and anatomical substrates of the amphetamine response in the rat. Brain Res. 1975;83:419–436. doi: 10.1016/0006-8993(75)90834-3. [DOI] [PubMed] [Google Scholar]
- de Rover M, Mansvelder HD, Lodder JC, Wardeh G, Schoffelmeer AN, Brussaard AB. Long-lasting nicotinic modulation of GABAergic synaptic transmission in the rat nucleus accumbens associated with behavioural sensitization to amphetamine. Eur J Neurosci. 2004;19:2859–2870. doi: 10.1111/j.0953-816X.2004.03370.x. [DOI] [PubMed] [Google Scholar]
- Devane WA, Hanus L, Breuer A, Pertwee RG, Stevenson LA, Griffin G, Gibson D, Mandelbaum A, Etinger A, Mechoulam R. Isolation and structure of a brain constituent that binds to the cannabinoid receptor. Science. 1992;258:1946–1949. doi: 10.1126/science.1470919. [DOI] [PubMed] [Google Scholar]
- Di Marzo V, Bisogno T, De Petrocellis L. Anandamide: some like it hot. Trends Pharmacol Sci. 2001;22:346–349. doi: 10.1016/s0165-6147(00)01712-0. [DOI] [PubMed] [Google Scholar]
- Felder CC, Briley EM, Axelrod J, Simpson JT, Mackie K, Devane WA. Anandamide, an endogenous cannabimimetic eicosanoid, binds to the cloned human cannabinoid receptor and stimulates receptor-mediated signal transduction. Proc Natl Acad Sci U S A. 1993;90:7656–7660. doi: 10.1073/pnas.90.16.7656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferrari F, Ottani A, Giuliani D. Influence of the cannabinoid agonist HU 210 on cocaine- and CQP 201-403-induced behavioural effects in rat. Life Sci. 1999;65:823–831. doi: 10.1016/s0024-3205(99)00309-4. [DOI] [PubMed] [Google Scholar]
- Filip M, Gołda A, Zaniewska M, McCreary AC, Nowak E, Kolasiewicz W, Przegaliński E. Involvement of cannabinoid CB1 receptors in drug addiction: effects of rimonabant on behavioral responses induced by cocaine. Pharmacol Rep. 2006;58:806–819. [PubMed] [Google Scholar]
- Fride E, Mechoulam R. Pharmacological activity of the cannabinoid receptor agonist, anandamide, a brain constituent. Eur J Pharmacol. 2006;231:313–314. doi: 10.1016/0014-2999(93)90468-w. [DOI] [PubMed] [Google Scholar]
- Hejazi N, Zhou C, Oz M, Sun H, Ye JH, Zhang L. Delta9-tetrahydrocannabinol and endogenous cannabinoid anandamide directly potentiate the function of glycine receptors. Mol Pharmacol. 2006;69:991–997. doi: 10.1124/mol.105.019174. [DOI] [PubMed] [Google Scholar]
- Herkenham M, Lynn AB, de Costa BR, Richfield EK. Neuronal localization of cannabinoid receptors in the basal ganglia of the rat. Brain Res. 1991;547:267–274. doi: 10.1016/0006-8993(91)90970-7. [DOI] [PubMed] [Google Scholar]
- Järbe TU, DiPatrizio NV, Li C, Makriyanni s A. The cannabinoid receptor antagonist SR-141716 does not readily antagonize open-field effects induced by the cannabinoid receptor agonist (R)-methanandamide in rats. Pharmacol Biochem Behav. 2003;75:809–821. doi: 10.1016/s0091-3057(03)00168-0. [DOI] [PubMed] [Google Scholar]
- Kalivas PW, Stewart J. Dopamine transmission in the initiation and expression of drug- and stress-induced sensitization of motor activity. Brain Res Brain Res Rev. 1991;16:223–244. doi: 10.1016/0165-0173(91)90007-u. [DOI] [PubMed] [Google Scholar]
- Kauer JA, Malenka RC. Synaptic plasticity and addiction. Nat Rev Neurosci. 2007;8:844–858. doi: 10.1038/nrn2234. [DOI] [PubMed] [Google Scholar]
- Kelly PH, Seviour PW, Iversen SD. Amphetamine and apomorphine responses in the rat following 6-OHDA lesions of the nucleus accumbens septi and corpus striatum. Brain Res. 1975;94:507–522. doi: 10.1016/0006-8993(75)90233-4. [DOI] [PubMed] [Google Scholar]
- Lee J, Di Marzo V, Brotchie JM. A role for vanilloid receptor 1 (TRPV1) and endocannabinnoid signalling in the regulation of spontaneous and L-DOPA induced locomotion in normal and reserpine-treated rats. Neuropharmacology. 2006;51:557–565. doi: 10.1016/j.neuropharm.2006.04.016. [DOI] [PubMed] [Google Scholar]
- Lesscher HM, Hoogveld E, Burbach JP, van Ree JM, Gerrits MA. Endogenous cannabinoids are not involved in cocaine reinforcement and development of cocaine-induced behavioural sensitization. Eur Neuropsychopharmacol. 2005;15:31–37. doi: 10.1016/j.euroneuro.2004.04.003. [DOI] [PubMed] [Google Scholar]
- Lozovaya N, Yatsenko N, Beketov A, Tsintsadze T, Burnashev N. Glycine receptors in CNS neurons as a target for nonretrograde action of cannabinoids. J Neurosci. 2005;25:7499–7506. doi: 10.1523/JNEUROSCI.0977-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maingret F, Patel AJ, Lazdunski M, Honoré E. The endocannabinoid anandamide is a direct and selective blocker of the background K(+) channel TASK-1. EMBO J. 2001;20:47–54. doi: 10.1093/emboj/20.1.47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malinowska B, Kwolek G, Göthert M. Ana ndamide and methanandamide induce both vanilloid VR1- and cannabinoid CB1 receptor-mediated changes in heart rate and blood pressure in anaesthetized rats. Naunyn Schmiedebergs Arch Pharmacol. 2001;364:562–569. doi: 10.1007/s00210-001-0498-6. [DOI] [PubMed] [Google Scholar]
- Mechoulam R, Fride E, Di Marzo V. Endocannabinoids. Eur J Pharmacol. 1998;359:1–18. doi: 10.1016/s0014-2999(98)00649-9. [DOI] [PubMed] [Google Scholar]
- Mezey E, Tóth ZE, Cortright DN, Arzubi MK, Krause JE, Elde R, Guo A, Blumberg PM, Szallasi A. Distribution of mRNA for vanilloid receptor subtype 1 (VR1), and VR1-like immunoreactivity, in the central nervous system of the rat and human. Proc Natl Acad Sci U S A. 2000;97:3655–3660. doi: 10.1073/pnas.060496197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moss DE, Koob GF, McMaster SB, Janowsky DS. Comparative effects of tetrahydrocannabinol on psychostimulant-induced behaviors. Pharmacol Biochem Behav. 1984;21:641–644. doi: 10.1016/s0091-3057(84)80050-7. [DOI] [PubMed] [Google Scholar]
- Muschamp JW, Siviv SM. Behavioral sensitization to amphetamine follows chronic administration of the CB1 agonist WIN 55,212-2 in Lewis rats. Pharmacol Biochem Behav. 2002;73:835–842. doi: 10.1016/s0091-3057(02)00910-3. [DOI] [PubMed] [Google Scholar]
- Narendran R, Martinez D. Cocaine abuse and sensitization of striatal dopamine transmission: a critical review of the preclinical and clinical imaging literature. Synapse. 2008;62:851–869. doi: 10.1002/syn.20566. [DOI] [PubMed] [Google Scholar]
- Nicholson RA, Liao C, Zheng J, David LS, Coyne L, Errington AC, Singh G, Lees G. Sodium channel inhibition by anandamide and synthetic cannabimimetics in brain. Brain Res. 2003;978:194–204. doi: 10.1016/s0006-8993(03)02808-7. [DOI] [PubMed] [Google Scholar]
- Oz M, Zhang L, Ravindran A, Morales M, Lupica CR. Differential effects of endogenous and synthetic cannabinoids on alpha7-nicotinic acetylcholine receptor-mediated responses in Xenopus Oocytes. J Pharmacol Exp Ther. 2004;310:1152–1160. doi: 10.1124/jpet.104.067751. [DOI] [PubMed] [Google Scholar]
- Oz M, Ravindran A, Diaz-Ruiz O, Zhang L, Morales M. The endogenous cannabinoid anandamide inhibits alpha7 nicotinic acetylcholine receptor-mediated responses in Xenopus oocytes. J Pharmacol Exp Ther. 2003;306:1003–1010. doi: 10.1124/jpet.103.049981. [DOI] [PubMed] [Google Scholar]
- Paulson PE, Robinson TE. Amphetamine-induced time-dependent sensitization of dopamine neurotransmission in the dorsal and ventral striatum: a microdialysis study in behaving rats. Synapse. 1995;19:56–65. doi: 10.1002/syn.890190108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paulson PE, Camp DM, Robinson TE. Time course of transient behavioral depression and persistent behavioral sensitization in relation to regional brain monoamine concentrations during amphetamine withdrawal in rats. Psychopharmacology(Berl) 1991;103:480–492. doi: 10.1007/BF02244248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pearlman RJ, Aubrey KR, Vandenberg RJ. Arachidonic acid and anandamide have opposite modulatory actions at the glycine transporter, GLYT1a. J Neurochem. 2003;84:592–601. doi: 10.1046/j.1471-4159.2003.01549.x. [DOI] [PubMed] [Google Scholar]
- Pertwee RG. Pharmacology of cannabinoid CB1 and CB2 receptors. Pharmacol Ther. 1997;74:129–180. doi: 10.1016/s0163-7258(97)82001-3. [DOI] [PubMed] [Google Scholar]
- Pierce RC, Kalivas PW. A circuitry model of the expression of behavioral sensitization to amphetamine-like psychostimulants. A circuitry model of the expression of behavioral sensitization to amphetamine-like psychostimulants. Brain Res Brain Res Rev. 1997;25:192–216. doi: 10.1016/s0165-0173(97)00021-0. [DOI] [PubMed] [Google Scholar]
- Przegaliński E, Göthert M, Frankowska M, Filip M. WIN 55,212-2-induced reduction of cocaine hyperlocomotion: possible inhibition of 5-HT(3) receptor function. Eur J Pharmacol. 2005;517:68–73. doi: 10.1016/j.ejphar.2005.05.014. [DOI] [PubMed] [Google Scholar]
- Przegaliński E, Siwanowicz J, Chojnacka-Wójcik E. Lack of effect of a glycine(B) receptor partial agonist on amphetamine-induced sensitization in mice. Pol J Pharmacol. 1999;51:385–390. [PubMed] [Google Scholar]
- Rasmussen BA, Kim E, Unterwald EM, Rawls SM. Methanandamide attenuates cocaine-induced hyperthermia in rats by a cannabinoid CB1-dopamine D2 receptor mechanism. Brain Res. 2009 doi: 10.1016/j.brainres.2008.12.078. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts LA, Ross HR, Connor M. Methanandamide activation of a novel current in mouse trigeminal ganglion sensory neurons in vitro. Neuropharmacology. 2008;54:172–180. doi: 10.1016/j.neuropharm.2007.05.024. [DOI] [PubMed] [Google Scholar]
- Robinson TE, Browman KE, Crombag HS, Badiani A. Modulation of the induction or expression of psychostimulant sensitization by the circumstances surrounding drug administration. Neurosci Biobehav Rev. 1998;22:347–354. doi: 10.1016/s0149-7634(97)00020-1. [DOI] [PubMed] [Google Scholar]
- Robinson TE, Berridge KC. The neural basis of drug craving: an incentive-sensitization theory of addiction. Brain Res Rev. 1993;18:247–291. doi: 10.1016/0165-0173(93)90013-p. [DOI] [PubMed] [Google Scholar]
- Robinson TE, Camp DM. Long-lasting effects of escalating doses of d-amphetamine on brain monoamines, amphetamine-induced stereotyped behavior and spontaneous nocturnal locomotion. Pharmacol Biochem Behav. 1987;26:821–827. doi: 10.1016/0091-3057(87)90616-2. [DOI] [PubMed] [Google Scholar]
- Romero J, García-Palomero E, Lin SY, Ramos JA, Makriyannis A, FernándezRuiz JJ. Extrapyramidal effects of methanandamide, an analog of anandamide, the endogenous CB1 receptor ligand. Life Sci. 1996;58:1249–1257. doi: 10.1016/0024-3205(96)00086-0. [DOI] [PubMed] [Google Scholar]
- Romero J, García L, Cebeira M, Zadrozny D, Fernández-Ruiz JJ, Ramos JA. The endogenous cannabinoid receptor ligand, anandamide, inhibits the motor behavior: role of nigrostriatal dopaminergic neurons. Life Sci. 1995;56:2033–2040. doi: 10.1016/0024-3205(95)00186-a. [DOI] [PubMed] [Google Scholar]
- Schoffelmeer AN, De Vries TJ, Wardeh G, van de Ven HW, Vanderschuren LJ. Psychostimulant-induced behavioral sensitization depends on nicotinic receptor activation. J Neurosci. 2002;22:3269–3276. doi: 10.1523/JNEUROSCI.22-08-03269.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seiden LS, Sabol KE, Ricaurte GA. Amphetamine: effects on catecholamine systems and behavior. Annu Rev Pharmacol Toxicol. 1993;33:639–677. doi: 10.1146/annurev.pa.33.040193.003231. [DOI] [PubMed] [Google Scholar]
- Soderman AR, Unterwald EM. Cocaine reward and hyperactivity in the rat: Sites of mu opioid receptor modulation. Neuroscience. 2008;154:1506–1516. doi: 10.1016/j.neuroscience.2008.04.063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thiemann G, Di Marzo V, Molleman A, Hasenöhrl RU. The CB(1) cannabinoid receptor antagonist AM251 attenuates amphetamine-induced behavioural sensitization while causing monoamine changes in nucleus accumbens and hippocampus. Pharmacol Biochem Behav. 2008a;89:384–391. doi: 10.1016/j.pbb.2008.01.010. [DOI] [PubMed] [Google Scholar]
- Thiemann G, van der Stelt M, Petrosino S, Molleman A, Di Marzo V, Hasenöhrl RU. The role of the CB1 cannabinoid receptor and its endogenous ligands, anandamide and 2-arachidonoylglycerol, in amphetamine-induced behavioural sensitization. Behav Brain Res. 2008b;187:289–296. doi: 10.1016/j.bbr.2007.09.022. [DOI] [PubMed] [Google Scholar]
- Vanderschuren LJ, Kalivas PW. Alterations in dopaminergic and glutamatergic transmission in the induction and expression of behavioral sensitization: a critical review of preclinical studies. Psychopharmacology (Berl) 2000;151:99–120. doi: 10.1007/s002130000493. [DOI] [PubMed] [Google Scholar]
- Vezina P. Sensitization of midbrain dopamine neuron reactivity and the self-administration of psychomotor stimulant drugs. Neurosci Biobehav Rev. 2004;27:827–839. doi: 10.1016/j.neubiorev.2003.11.001. [DOI] [PubMed] [Google Scholar]
- Wenger T, Moldrich G, Furst S. Neuromorphological background of cannabis addiction. Brain Res Bull. 2003;61:125–128. doi: 10.1016/s0361-9230(03)00081-9. [DOI] [PubMed] [Google Scholar]
- Zygmunt PM, Petersson J, Andersson DA, Chuang H, Sørgård M, Di Marzo V, Julius D, Högestätt ED. Vanilloid recep tors on sensory nerves mediate the vasodilator action of anandamide. Nature. 1999;400:452–457. doi: 10.1038/22761. [DOI] [PubMed] [Google Scholar]