Abstract
Portopulmonary hypertension (POPH) and hepatopulmonary syndrome (HPS) are two frequent complications of liver disease, with prevalence among liver transplant candidates of 6% and 10%, respectively. Both conditions result from a lack of hepatic clearance of vasoactive substances produced in the splanchnic territory. Subsequently, these substances cause mainly pulmonary vascular remodeling and some degree of vasoconstriction in POPH with resulting elevated pulmonary pressure and right ventricular dysfunction. In HPS the vasoactive mediators cause intrapulmonary shunts with hypoxemia. Medical treatment is disappointing overall. Whereas liver transplantation (LT) results in the disappearance of HPS within six to twelve months, its effect on POPH is highly unpredictable. Modern strategies in managing HPS and POPH rely on a thorough screening and grading of the disease’s severity, in order to tailor the appropriate therapy and select only the patients who will benefit from LT. The anesthesiologist plays a central role in managing these high-risk patients. Indeed, the important hemodynamic and respiratory modifications of the perioperative period must be avoided through continuation of the preoperatively initiated drugs, appropriate intraoperative monitoring and proper hemodynamic and respiratory therapies.
Keywords: End stage liver disease, Hepatopulmonary syndrome, Portopulmonary Hypertension, Anesthesia
Core tip: Portopulmonary hypertension (POPH) and hepatopulmonary syndrome (HPS) are frequent complications of liver disease. Both conditions result from diminished hepatic clearance of splanchnic vasoactive substances. They cause pulmonary vasoconstriction in POPH resulting in elevated pulmonary pressure, right ventricular dysfunction and intrapulmonary shunts with hypoxemia in HPS. The only lasting treatment is liver transplantation (LT). Whereas LT results in the disappearance of HPS, its effect on POPH is unpredictable. The anesthesiologist plays a central role in managing HPS and POPH during LT as preoperative screening and grading of the disease allows the selection of appropriate therapies.
INTRODUCTION
Liver transplantation (LT) has been performed since 1963 and is now a worldwide procedure. It has cured many patients, whatever the liver disease etiology. This intervention, which was deemed very high-risk in the first days of transplantation medicine, nowadays carries a 30-d and 5-year mortality rate of 5% and 30%, respectively. This is comparable to high-risk surgical procedures in high volume centers[1,2]. However, several problems remain, among which lay portopulmonary hypertension (POPH) and the hepatopulmonary syndrome (HPS). These liver disease complications make LT a riskier procedure, reduce its chances of success, worsen the overall patient’s prognosis and represent a genuine challenge to the anesthesiologist[3,4]. In the past years, light has been shed on the epidemiology, pathophysiology and treatment of POPH and HPS. We will hereby review the latest trends in POPH and HPS management; in particular in the anesthesiologist’s view.
Portopulmonary hypertension
POPH is the association between pulmonary hypertension and portal hypertension with or without hepatic disease. Indeed, portal hypertension per se can induce pulmonary hypertension. Interestingly, approximately 10% of POPH patients have portal hypertension without cirrhosis. This picture is found most frequently in patients infected with Schistosoma mansoni[5]. Furthermore, the development of pulmonary hypertension is not related to the severity of liver disease. It is defined by portal hypertension, mean pulmonary artery pressure (mPAP) > 25 mmHg, pulmonary vascular resistance (PVR) > 240 dyn.s.cm-5 and pulmonary artery occlusion pressure (mPAOP) < 15 mmHg (Table 1)[6,7]. The pathophysiology of POPH remains unclear: it is observed in cirrhotic and non-cirrhotic portal hypertension and is related to neither the etiology of liver disease nor the severity of portal hypertension. However, female sex and autoimmune disease are important risk factors[8]. POPH has the same features of plexogenic arteriopathy of idiopathic pulmonary hypertension involving endothelial and smooth muscle proliferation. It has been also suggested that hyperkinetic status could promote shear stress and induce endothelial dysfunction. Moreover, inflammatory condition observed in patients with cirrhosis (endotoxemia, bacterial translocation) could participate to the pulmonary vascular remodeling. Another widely accepted explanation is that mediators produced in the splanchnic circulation and normally metabolized by the liver reach the pulmonary circulation through portosystemic collaterals with subsequent injury to pulmonary vessels. Putative harmful mediators are serotonin and endothelin, increased plasma concentrations of these mediators being identified in patients with portal hypertension. Besides their vasoconstrictive effect, these mediators also promote cell proliferation[9]. Nevertheless, despite sophisticated testing, the pathogenesis of POPH remains elusive.
Table 1.
Diagnostic criteria of portopulmonary hypertension
| Criteria 1 |
| Portal hypertension (15 mmHg, or portocaval gradient > 5 mmHg) and |
| Criteria 2 |
| mPAP > 25 mmHg and mPAOP < 15 mmHg |
| mPAP - mPAOP (transpulmonary gradient) > 10 mmHg |
| PVR > 240 dyn.s.cm-5 = 3 UI WOOD |
mPAP: Mean pulmonary artery pressure; mPAOP: Mean pulmonary artery occlusion pressure; PVR: Pulmonary vascular resistance.
POPH is a relatively common condition among LT candidates with a prevalence of approximately 6%[10,11]. Likewise, it worsens their prognosis. Their outcome is poor with a one-year survival of approximately 85%[12,13] and three-year survival varying between 38% and 68%[13]. Finally, a large American cohort revealed a five-year survival of only 40%[14]. The differences in survival rates between these trials can probably be explained in part by the severity of the underlying liver diseases. For instance, in the study by Le Pavec, which showed the best outcome, there was a large proportion of Child Pugh class A cirrhotic patients (51%). Furthermore, it is well established that POPH per se, depending on its severity, has a negative impact on LT success.
It is now clear from these findings that patients with POPH should be properly diagnosed preoperatively, to initiate the right treatment promptly and select exclusively those with a non-prohibitive level of POPH for LT (Tables 2, 3 and Figure 1). POPH’s presentation ranges from totally asymptomatic to severe dyspnea, fatigue and peripheral edema. As it can be asymptomatic, POPH should be sought in every LT candidate. Investigations such as ECG, chest X-ray, blood gas analysis and lung function testing have a very low diagnostic yield[15]. In most centers, transthoracic echocardiography (TTE) is performed as a screening test, owing to its high sensitivity and non-invasive nature. It is able to reliably exclude POPH in most POPH-free patients. Different centers use various cut-off values of pulmonary artery systolic pressure (PASP) ranging from 30 to 50 mmHg[16]. For instance, PASP > 50 mmHg has sensitivity and specificity of 97 and 77%, respectively[17]. However, the best cut-off value to avoid undiagnosed POPH and prevent unnecessary right heart catheterization (RHC) remains undetermined. Another important feature of transesophageal echocardiography (TEE) is its ability to rule out other causes of elevated pulmonary arterial pressure such as left heart dysfunction. The next step after TEE in patients with elevated PASP is RHC. Cardiac output (CO), mPAP, mPAOP and PVR can help to determine the nature and severity of the POPH. RHC can ascertain the diagnosis of POPH and exclude other frequently encountered causes of pulmonary hypertension in the LT candidate. Indeed, there are three main causes of elevated mPAP in liver disease patients: cirrhotic cardiomyopathy (left ventricular dysfunction), the typical high-output state of cirrhosis and POPH. For instance, in the hyperdynamic state, CO and mPAP are elevated, mPAOP is normal and PVR is decreased owing to a passive dilation of pulmonary vessels. In left ventricular dysfunction, CO is reduced; PVR, mPAP and mPAOP are elevated. Finally, in POPH, CO and mPAOP are low while PVR and mPAP are high (Table 2). It is very important to differentiate these conditions as their treatment and prognosis are entirely different. Furthermore, RHC is also used to carry out reversibility tests on the pulmonary vasculature with various vasodilators. However, even though the rate of acute responders varies in the literature from 1.3% to 43%[18,19], their long-term response to therapy cannot be predicted[20]. Thus, RHC is central to diagnosing and grading POPH (Table 3). The precise grading of POPH is of uttermost importance as treatment and prognosis vary dramatically according to the severity of the disease.
Table 2.
Hemodynamic patterns in cirrhotics with elevated mean pulmonary artery pressure
| Hemodynamic pattern | mPAP | PVR | mPAOP | CO |
| Hyperdynamic state | ↑ | ↓ | → | ↑↑ |
| Left heart dysfunction | ↑ | ↑ | ↑ | ↓ |
| POPH | ↑ | ↑ | → | ↓ |
Adapted from Machicao et al[15]. CO: Cardiac output; mPAOP: Mean pulmonary artery occlusion pressure; mPAP: Mean pulmonary artery pressure; POPH: Portopulmonary hypertension; PVR: Pulmonary vascular resistance.
Figure 1.
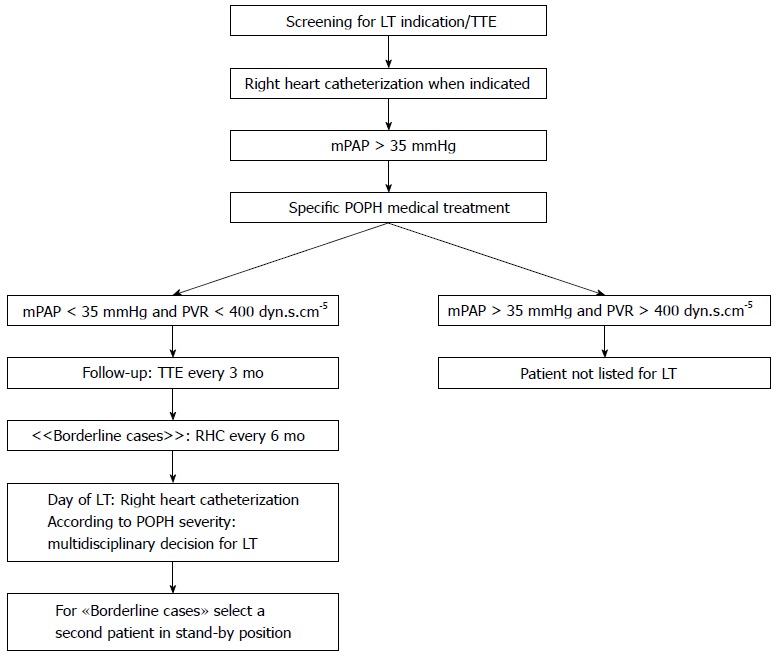
Portopulmonary hypertension screening and follow-up algorithm for liver transplantation candidates in Geneva. LT: Liver transplantation; mPAP: Mean pulmonary artery pressure; POPH: Portopulmonary hypertension; PVR: Pulmonary vascular resistance; TTE: Transthoracic doppler echocardiography.
Table 3.
Severity score of pulmonary hypertension in portopulmonary hypertension patients based on liver transplantation mortality
| Mild: 25 mmHg < mPAP < 35 mmHg |
| Moderate: 35 mmHg ≤ mPAP < 45 mmHg |
| Severe: mPAP ≥ 45 mmHg |
mPAP: Mean pulmonary artery pressure.
TREATMENT
In mild POPH, no treatment is warranted as several studies have shown that LT outcome is not different from POPH-free patients[8,21]. For moderate to severe POPH, LT is not the definitive treatment. In some patients, it disappears several months after LT, whereas in others it persists or even worsens over the course of time. The postoperative disease evolution is highly unpredictable, thus compromising the chances of LT success. Therefore, pulmonary vasodilators should be employed with the aim of lowering mPAP < 35 mmHg, to minimize the risk of graft failure and improve the overall outcome.
Swanson et al[22] demonstrated that when comparing patients without any treatment, with specific POPH therapy and with a combination of specific POPH therapy and LT, the latter group reached the best outcome. Five-year survival was 14%, 45% and 76%, respectively.
There are several different specific POPH therapies. All of which are derived from the treatment of idiopathic pulmonary hypertension (Table 4)[23].
Table 4.
Portopulmonary hypertension treatments[69]
| Molecule | Mechanism of action | Half-life | Administration | Adverse effects | Advantages | Drawbacks | Main results |
| Nitric Oxide | Selective dilation of the pulmonary vasculature | Seconds | Inhaled | Methemoglobinemia | Selective on pulmonary circulation | Endotracheal intubation | Improved hemodynamics |
| Epoprostenol | Vasodilation of all vascular beds/platelet inhibition | Minutes | Intravenous | Flushing, headache, nausea | Best studied drug | Long term central venous access/cost | Improved hemodynamics allowing LT[70,71] |
| Iloprost | Vasodilation of pulmonary vascular bed/platelet inhibition | Minutes | Inhaled | Flushing, headache, nausea | Selective on pulmonary circulation | Frequent administration/cost | Increased exercise tolerance and survival[26,27,30] |
| Bosentan | Endothelin receptor antagonist | 5 h | Oral | Hepatotoxicity, peripheral edema | Oral administration, twice daily | Cost | Improved survival[29] |
| Sildenafil | Phosphodiesterase type 5 inhibitor | 4 h | Oral | Flushing, headache, priapism | Oral administration, three times daily | Cost | Increased exercise tolerance[31] |
LT: Liver transplantation.
Nitric oxide (NO) is a selective pulmonary vasculature dilator when used in the inhaled form. It is effective but almost always necessitates an intubated patient for an accurate administration. Indeed, without an endotracheal tube, its concentration cannot be titrated properly. Therefore, its use is mainly restricted to the operating room and ICU, in the setting of acute right ventricular failure due to POPH worsening.
Epoprostenol, a prostacyclin, which is used parenterally, is a vasodilator, a platelet inhibitor, and has an antiproliferative effect. In two recent studies[24,25], the administration of epoprostenol in moderate to severe POPH was associated with a drop in mPAP < 35 mmHg in a majority of patients (75%-88%), which allowed considering LT. Its main adverse effect is thrombocytopenia.
Iloprost is an inhaled prostacyclin, which improves exercise tolerance and causes pulmonary vasculature dilation[26,27]. A recent study showed that survival was improved when parenteral prostacyclin was started precociously[28].
Bosentan is an endothelin receptor antagonist that has been shown to improve survival as compared to iloprost[29,30].
Finally, sildenafil, a phosphodiesterase inhibitor, improves exercise capacity[31].
Anticoagulants are not indicated as in idiopathic pulmonary hypertension because of the major bleeding disorder associated with end-stage liver disease. Furthermore, beta blockers are contra-indicated in POPH, as they worsen pulmonary hemodynamics and cardiopulmonary reserve[32].
All these treatments have mainly been validated in patients with idiopathic pulmonary hypertension, which is a different disease from POPH. They have their limitations and must be chosen on an individual basis, taking into account their various adverse effects (Table 4). The various treatment recommendations come from small retrospective trials with inherent limitations. A confirmation by randomized controlled trials is needed. Finally, one must bear in mind that these treatments are mainly useful lowering mPAP and make LT feasible.
On a practical point of view, after patients with moderate and severe POPH (mPAP > 35 mmHg, PVR > 240 dyn.s.cm-5) have been started on pulmonary vasodilators, their response to treatment is assessed by TTE and RHC in borderline cases every three months. If mPAP remains over 35 mmHg, LT is contra-indicated. If the patient is responsive to treatment (mPAP < 35 mmHg) MELD exception points are assigned and LT can be considered, as long as the PVR remains under 400 dyn.s.cm-5 and the right ventricular function is preserved[15].
ANESTHETIC MANAGEMENT CONSIDERATIONS
In our center, LT candidates with known moderate to severe POPH, confirmed by RHC, are started on a pulmonary vasodilator as soon as the diagnosis is made. On the day of LT, a RHC is performed in the anesthesia suite and, the decision whether or not to proceed with LT is taken by a multidisciplinary team. Furthermore, a second recipient is called and asked to remain in stand-by in case of a cancelled LT (Figure 1).
During the intraoperative period, various factors may contribute to the increase in mPAP, which may then lead to graft failure and right ventricular failure. The anesthesiologist, through the use of the appropriate hemodynamic monitoring, plays an essential role in the early diagnosis of worsening intraoperative POPH and its treatment.
The anesthesia induction is a critical phase. At the same moment, systemic vasomotor tone typically diminishes and positive pressure ventilation is initiated. This can lead to worsening of POPH and right ventricular failure. Therefore, anesthesia induction must be as smooth as possible. Commonly used hypnotics are etomidate, midazolam and a combination of ketamine and propofol (ketofol), as they all preserve hemodynamic stability. Regarding ventilation, high levels of ventilation pressure and positive end expiratory pressure should be avoided, as well as hypoxia, hypercapnia and acidosis, all of which aggravate POPH.
The patient should be monitored with an arterial line, a pulmonary artery catheter (PAC) and possibly a TEE. The PAC is commonly used in LT. In POPH, this advanced hemodynamic monitor is particularly useful as it can measure CO and PAP precisely and help the anesthesiologist in correct decision-making[33]. For instance, the occurrence of a decompensated POPH (normal-high mPAP, high PVR, low CO) will be recognized and treated differently from hypovolemia, another frequently encountered situation in LT, (low mPAP, low CO). Moreover, its use is mandatory for postoperative care.
Some authors recommend TEE as the best hemodynamic monitor in POPH patients. It allows real-time measurement of PAP and is the best mean for evaluating right ventricular function. Of course, only an anesthesiologist properly trained in TEE must use it[34,35].
TEE should be considered cautiously in patients with esophageal varices because of the bleeding risk. However, esophageal varices are not a formal contraindication to TEE and the decision to use this monitor must be made on an individual basis, taking into account risk and benefit.
During the pre-anhepatic phase, major volume shifts can occur. The large quantities of crystalloids, colloids and blood products can cause an increase in POPH and central venous pressure (CVP).
Later on, during the anhepatic phase, the standard procedure consists of cross-clamping the inferior vena cava, portal vein and hepatic artery[36]. This causes a dramatic decrease in CO (40%-50%). The resulting effect in patients with POPH is unpredictable. However, hemodynamics can be dangerously worsened. Therefore, in our institution, we proceed with a cross-clamping algorithm (Figure 2), whereby a drop in mixed venous oxygen saturation (SVO2) below 70% warrants a venovenous bypass.
Figure 2.
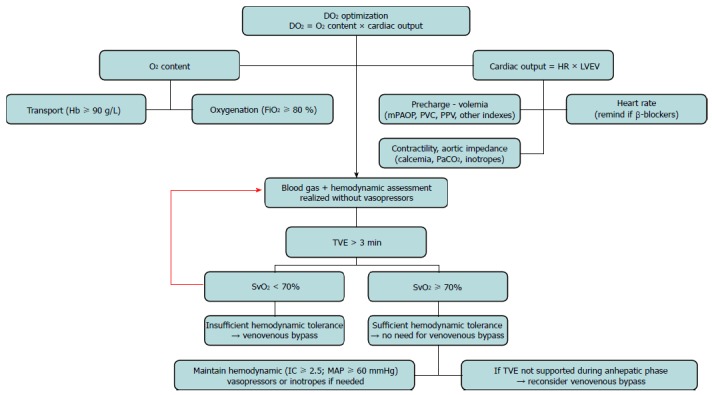
Challenge algorithm to total vascular exclusion of the liver. DO2: Oxygen delivery; Hb: Hemoglobin; FiO2: Inspired oxygen fraction; HR: Heart rate; LVEV: Left ventricular ejection volume; PaCO2: Partial pressure of carbon dioxide; TVE: Total vascular exclusion of the liver; SvO2: Mixed venous oxygen blood saturation.
After cross-clamp release, graft reperfusion occurs and several vasoactive substances (from anaerobic metabolism and cold preservation solutions) are released and tend to cause a peak in mPAP. This is known as the “reperfusion syndrome”. The use of a technique which produce less ischemia during the anhepatic phase, such as the “piggy-back technique” or use of a venovenous bypass, will avoid the surge in mPAP, thus being of particular interest in POPH patients[37]. Moreover, the reperfusion is accompanied by an increase in CO, which in turn raises mPAP.
In short, in the intraoperative period, mPAP can be increased in a dangerous way, leading to high CVP, graft congestion and malfunction, increased work of the right ventricle and finally right ventricular failure[38,39]. In the event of an elevated intraoperative PAP, and after having excluded other causes of pulmonary hypertension, the anesthesiologist can use pulmonary vasodilators such as NO and epoprostenol. If there is concomitant right heart failure, milrinone is recommended[33]. As milrinone is both an inotrope and a vasodilator, a vasopressor infusion (norepinephrine, phenylephrine) should be administered at the same time to prevent hypotension. It seems that giving a pulmonary vasodilator only intraoperatively is insufficient. It should be started preoperatively to improve outcome as it probably acts on vascular remodeling[40]. Various intraoperative prognostic factors have been associated with adverse outcome, such as mean arterial pressure < 40 mmHg and mPAP > 40 mmHg[41].
The course of the disease following LT is highly unpredictable. Some patients develop refractory POPH with right ventricular failure. Therefore, the postoperative period is critical. The resolution of POPH is not systematic and medical treatment should not be discontinued until PAP has normalized.
Hepatopulmonary syndrome
The hepatopulmonary syndrome (HPS) is defined by the combination of intrapulmonary vascular dilatation (IPVD) and hypoxemia in patients with chronic liver disease or portal hypertension (Table 5)[6]. IPVD can cause a right to left shunt resulting in an elevated alveolar-arterial oxygen pressure gradient (A-aDO2) and hypoxemia. It seems that in presence of HPS, there is an imbalance in the vasoactive substances favoring vasodilatation. Furthermore, the mechanism of pulmonary hypoxic vasoconstriction is inhibited[42].
Table 5.
Diagnostic criteria of hepatopulmonary syndrome
| Criteria 1: Chronic liver disease |
| Criteria 2: A-aDO2 ≥ 15 mmHg[7,72], or ≥ 20 mmHg[52], or ≥ to the age-adjusted value1[73] |
| Criteria 3: intrapulmonary vascular dilatation at CE-TTE[7] or 99mTcMAA[7] |
1Age-adjusted value: 0.26 (age) - 0.43. A-aDO2: Alveolar-arterial oxygen pressure gradient; CE-TTE: Contrast-enhanced echocardiography;
TcMAA: Technetium macroaggregated albumin lung perfusion scan.
IPVD are found in 40%-60% of liver disease patients, though only 15%-30% have associated hypoxemia and meet the diagnostic criteria of HPS[43]. Moreover, a study from our center showed a prevalence of 10% in LT candidates[44]. HPS is a well recognized cause of worsened outcome in the liver disease patient. Therefore, its early diagnosis is very important. Patients with HPS can present with dyspnea and platypnea due to orthodeoxia, although most of them are asymptomatic[45]. Therefore, HPS must be actively sought in every LT candidate. In patients with liver disease and hypoxemia, two tests can detect a shunt and establish the HPS diagnosis: contrast-enhanced transthoracic echocardiography (CE-TTE) and Technetium macroaggregated albumin lung perfusion scan (99mTc MAA scan). During CE-TTE, agitated saline is injected intravenously. In the occurrence of HPS, microbubbles will be visualized going from the right to the left atrium. CE-TTE allows for the distinction between intracardiac and intrapulmonary shunts. Indeed, in an intracardiac shunt, the bubbles are seen in the left atrium within three heartbeats, whereas in an intrapulmonary shunt, they are visualized within 4-6 beats. CE-TTE is a valuable test as it is non-invasive and very sensitive. The 99mTc MAA scan is the intravenous injection of radiolabeled albumin. It detects the existence of a shunt, but does not differentiate an intracardiac from an intrapulmonary shunt. However, it is useful in patients with concomitant respiratory disease as it can determine whether hypoxemia results from a shunt or a pulmonary pathology. A shunt fraction over 6% establishes that HPS is the main contributing factor to hypoxemia[40]. 99mTc MAA scan is therefore very valuable as 20%-30% of HPS patients suffer from concomitant respiratory disease[4,46]. Pulmonary angiography is seldom indicated. Nonetheless it can reveal an arteriovenous fistula which in some instances can be coiled[47].
Arterial blood gas analysis is of uttermost importance. It contributes to the diagnosis of HPS (hypoxemia, increased A-aDO2) and most importantly to HPS severity grading (Tables 5 and 6).
Table 6.
Severity score of hepatopulmonary syndrome[3]
| Stages | PaO2, mmHg |
| Mild | ≥ 80 |
| Moderate | ≥ 60 and < 80 |
| Severe | ≥ 50 and < 60 |
| Very severe | < 50 |
Other tests such as pulmonary function tests (PFT) and chest X-ray are neither sensitive nor specific and are of limited value in the diagnosis of HPS, although they can be useful in searching an alternative cause of hypoxemia. Apart from a reduced carbon monoxide diffusing capacity, PFT are usually normal.
We have proposed an algorithm for screening and grading HPS (Figure 3)[48]. The first step is to noninvasively measure the hemoglobin oxygen saturation (SpO2) by pulse oximetry, then analyze arterial blood gas and finally perform CE-TTE. Pulse oximetry, when taking a cut-off value of SpO2 < 96% has a sensitivity of 100% for the detection of PaO2 < 70 mmHg and is therefore an extremely effective screening test[49-51]. Arterial blood gas analysis yields the values of PaO2 and A-aDO2, thereby contributing to the diagnosis and grading of HPS. Finally CE-TTE ascertains the diagnosis by revealing the presence of IPVD (Table 5).
Figure 3.
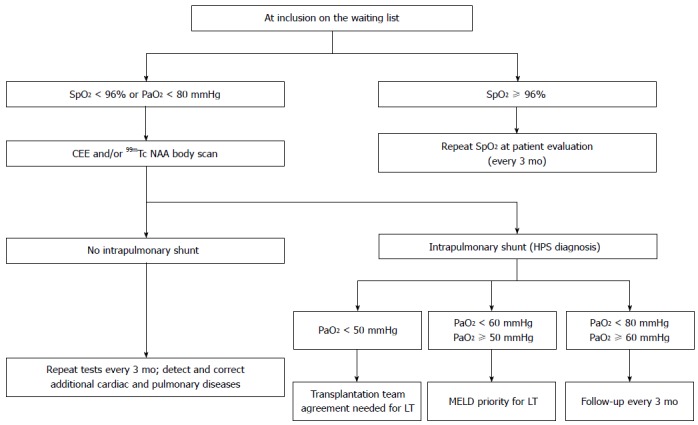
Hepatopulmonary syndrome screening, grading and follow-up algorithm for liver transplantation candidates. Adapted from Pastor et al[48]. SpO2: Hemoglobin oxygen saturation; HPS: Hepatopulmonary syndrome; PaO2: Partial pressure of oxygen; MELD: Model for end-stage liver disease; LT: Liver transplantation; 99mTc NAA: Technetium macroaggregated albumin.
Patients with mild to moderate HPS must be followed on a regular base, with arterial blood gas analysis every six months, as HPS tends to become severe over time[52]. On the other hand, patients diagnosed with severe HPS receive MELD exception points and are thereby prioritized in the LT waiting list[3,53]. The time until LT is usually less than three months under these circumstances. Finally, there is conflicting evidence that very severe HPS is a contraindication to LT as these patients have a high risk perioperative course. There is no consensus on whether or not to carry out LT in these patients; the decision depends on each center’s policy[20].
Overall, the medical treatment of HPS is disappointing. Various drugs, such as somatostatin, almitrin, indomethacin, NO, aspirin and beta blockers have been tested with no noticeable improvement[6]. Pentoxifylline has shown conflicting results[54,55]. Inhaled iloprost has proven some benefit on hypoxemia[56]. Finally, oxygen therapy is indicated when PaO2 is < 60 mmHg[57].
The only definitive treatment of HPS is LT. Several studies have shown that HPS is cured or significantly improved in more than 85% cases within 6-12 mo after LT[52,58]. Without LT, the 5-year survival rate of HPS is decreased compared with HPS-free cirrhotic patients[52,59,60]. Even though perioperative mortality is increased in severe HPS, the 5-year survival rates (76%) are identical to HPS-free patients[52]. The main predictors of unfavorable outcome after LT are PaO2 < 50 mmHg and a 99mTc MAA scan showing a shunt fraction > 20%[61].
ANESTHETIC MANAGEMENT
Operative management of the patient with HPS can be challenging. Mandatory monitoring comprises an arterial line and PAC. Regular blood gas analysis can be performed throughout the intervention. Worsening hypoxemia, whatever the cause, will be detected. The PAC, through the measurement of SVO2, CO and mPAOP, assists the anesthesiologist in the hemodynamic management of the HPS patient. Frequently encountered situations in LT, such as hypoxia, hypovolemia or hypervolemia, can be dealt with, appropriately. TEE can be very helpful in patients with preoperatively undiagnosed HPS as a contrast-enhanced study can be performed in the operating theater. It can also serve as an advanced hemodynamic monitoring, helping in fluid therapy and initiation of inotropes and vasopressors.
The anesthesia induction is critical in the HPS patient. In particular, a thorough preoxygenation must be done. Orotracheal intubation is performed in a rapid sequence and ventilation is started immediately. A steep drop in SpO2 can occur rapidly after anesthesia induction in patients with preexisting hypoxemia and ascites. Interestingly, preoxygenation, though highly recommended, is not always effective as the cause of hypoxemia is a true shunt.
Inhalational anesthesia seems to worsen hypoxemia more than intravenous agents, though the effect does not persist after one hour[62].
The lungs are ventilated using a protective strategy with a combination of low tidal volumes (6-8 mL/kg), a positive end expiratory pressure of 6-8 cm H2O and regular recruitment maneuvers[63]. This reduces the deleterious effects of mechanical ventilation and optimizes oxygenation.
During the pre-anhepatic phase, large fluid shifts occur and both hypovolemia and hypervolemia are observed. In our center, we use a goal-directed hemodynamic therapy, through the use of pulse pressure variation and the PAC-derived parameters, to optimize fluid, vasopressors and inotropes administration[64]. Indeed, in the case of HPS, both hypervolemia and hypovolemia have to be avoided. Hypervolemia leads to pulmonary edema and worsening hypoxemia, whereas hypovolemia compromises global oxygen delivery by reducing CO.
In our institution, we use a PAC with continuous SVO2 measurement for HPS. It allows for precise administration of oxygen and optimization of ventilation parameters. Moreover, it is useful to assess the patient’s tolerance to hepatic vascular clamping. If SVO2 falls under a value of 70%, a venovenous bypass is warranted. (Figure 2)[65].
POSTOPERATIVE CARE
One important fact to bear in mind in the postoperative period is that HPS does not resolve before several months after LT. Therefore, intraoperative and postoperative problems are similar. Fluid overload is deleterious but a frequently committed error is an overzealous forced diuresis. Of course, fluid overload compromises gas exchange and results in prolonged mechanical ventilation. However, hypovolemia leads to thick respiratory secretions, acute renal failure and multiple organ dysfunctions[20]. Therefore and once again, a tailored fluid therapy is of uttermost importance.
A particular concern in the postoperative care of HPS patients is hypoxemia. Indeed, hypoxemia can be aggravated after LT, due to various factors; i.e., atelectasis, fluid overload and capillary leak syndrome. These patients experience longer postoperative mechanical ventilation than HPS-free patients[46]. Apart from applying protective mechanical ventilation, different strategies have been tested to address the problem: frequent body positioning, inhaled NO, and in the most severe cases, venovenous extracorporeal membrane oxygenation[46,66,67]. Some authors recommend early extubation associated with immediate non-invasive ventilation and high-inspired fraction of oxygen, to avoid the harmful effects of mechanical ventilation as much as possible[20,68].
CONCLUSION
HPS and POPH are two relatively frequent complications of liver disease. Depending on their severity, they can obscure both the overall patients’ prognosis and the chances of LT success. Whereas HPS usually disappears within six to twelve months following LT, POPH tends to persist over time, thereby promoting graft failure and poor outcome.
It is therefore essential for these patients to be properly screened. Once the diagnosis of POPH or HPS has been established, the disease’s severity should be graded, the right treatment should be initiated and finally LT should be carried out only in a subset of patients who will benefit from it. The anesthesiologist carries a central role in all steps of the way. By selecting the right LT candidates and managing the major physiologic upsets of the perioperative period, the anesthesiologist can increase LT success rate. In recent years, the progress of perioperative medicine has brought a true improvement and cases that were deemed inoperable formerly, such as severe HPS, are now regularly performed. In the future, new drug treatments that target specific molecules involved in the pathophysiology of POPH and HPS constitute a promising research field. Also, indications and contraindications to LT, as well as exceptions to MELD score, should be regularly reassessed, based on the regular scrutiny of ever evolving data in liver disease research.
Footnotes
P- Reviewers: Degano B, Savale L S- Editor: Wen LL L- Editor: A E- Editor: Liu XM
References
- 1.Dienstag JL, Cosimi AB. Liver transplantation--a vision realized. N Engl J Med. 2012;367:1483–1485. doi: 10.1056/NEJMp1210159. [DOI] [PubMed] [Google Scholar]
- 2.Edwards EB, Roberts JP, McBride MA, Schulak JA, Hunsicker LG. The effect of the volume of procedures at transplantation centers on mortality after liver transplantation. N Engl J Med. 1999;341:2049–2053. doi: 10.1056/NEJM199912303412703. [DOI] [PubMed] [Google Scholar]
- 3.Fauconnet P, Klopfenstein CE, Schiffer E. Hepatopulmonary syndrome: the anaesthetic considerations. Eur J Anaesthesiol. 2013;30:721–730. doi: 10.1097/EJA.0b013e328365bb6f. [DOI] [PubMed] [Google Scholar]
- 4.Krowka MJ. Management of pulmonary complications in pretransplant patients. Clin Liver Dis. 2011;15:765–777. doi: 10.1016/j.cld.2011.08.012. [DOI] [PubMed] [Google Scholar]
- 5.Safdar Z, Bartolome S, Sussman N. Portopulmonary hypertension: an update. Liver Transpl. 2012;18:881–891. doi: 10.1002/lt.23485. [DOI] [PubMed] [Google Scholar]
- 6.Rodríquez-Roisin R, Krowka MJ, Hervé P, Fallon MB. Highlights of the ERS Task Force on pulmonary-hepatic vascular disorders (PHD) J Hepatol. 2005;42:924–927. doi: 10.1016/j.jhep.2005.03.002. [DOI] [PubMed] [Google Scholar]
- 7.Rodríguez-Roisin R, Krowka MJ, Hervé P, Fallon MB. Pulmonary-Hepatic vascular Disorders (PHD) Eur Respir J. 2004;24:861–880. doi: 10.1183/09031936.04.00010904. [DOI] [PubMed] [Google Scholar]
- 8.Kawut SM, Krowka MJ, Trotter JF, Roberts KE, Benza RL, Badesch DB, Taichman DB, Horn EM, Zacks S, Kaplowitz N, et al. Clinical risk factors for portopulmonary hypertension. Hepatology. 2008;48:196–203. doi: 10.1002/hep.22275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Michelakis ED, Wilkins MR, Rabinovitch M. Emerging concepts and translational priorities in pulmonary arterial hypertension. Circulation. 2008;118:1486–1495. doi: 10.1161/CIRCULATIONAHA.106.673988. [DOI] [PubMed] [Google Scholar]
- 10.Colle IO, Moreau R, Godinho E, Belghiti J, Ettori F, Cohen-Solal A, Mal H, Bernuau J, Marty J, Lebrec D, et al. Diagnosis of portopulmonary hypertension in candidates for liver transplantation: a prospective study. Hepatology. 2003;37:401–409. doi: 10.1053/jhep.2003.50060. [DOI] [PubMed] [Google Scholar]
- 11.Krowka MJ, Swanson KL, Frantz RP, McGoon MD, Wiesner RH. Portopulmonary hypertension: Results from a 10-year screening algorithm. Hepatology. 2006;44:1502–1510. doi: 10.1002/hep.21431. [DOI] [PubMed] [Google Scholar]
- 12.Kawut SM, Taichman DB, Ahya VN, Kaplan S, Archer-Chicko CL, Kimmel SE, Palevsky HI. Hemodynamics and survival of patients with portopulmonary hypertension. Liver Transpl. 2005;11:1107–1111. doi: 10.1002/lt.20459. [DOI] [PubMed] [Google Scholar]
- 13.Le Pavec J, Souza R, Herve P, Lebrec D, Savale L, Tcherakian C, Jaïs X, Yaïci A, Humbert M, Simonneau G, et al. Portopulmonary hypertension: survival and prognostic factors. Am J Respir Crit Care Med. 2008;178:637–643. doi: 10.1164/rccm.200804-613OC. [DOI] [PubMed] [Google Scholar]
- 14.Krowka MJ, Miller DP, Barst RJ, Taichman D, Dweik RA, Badesch DB, McGoon MD. Portopulmonary hypertension: a report from the US-based REVEAL Registry. Chest. 2012;141:906–915. doi: 10.1378/chest.11-0160. [DOI] [PubMed] [Google Scholar]
- 15.Machicao VI, Balakrishnan M, Fallon MB. Pulmonary complications in chronic liver disease. Hepatology. 2014;59:1627–1637. doi: 10.1002/hep.26745. [DOI] [PubMed] [Google Scholar]
- 16.Fritz JS, Fallon MB, Kawut SM. Pulmonary vascular complications of liver disease. Am J Respir Crit Care Med. 2013;187:133–143. doi: 10.1164/rccm.201209-1583CI. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Krowka MJ, Wiesner RH, Heimbach JK. Pulmonary contraindications, indications and MELD exceptions for liver transplantation: a contemporary view and look forward. J Hepatol. 2013;59:367–374. doi: 10.1016/j.jhep.2013.03.026. [DOI] [PubMed] [Google Scholar]
- 18.Krowka MJ, Frantz RP, McGoon MD, Severson C, Plevak DJ, Wiesner RH. Improvement in pulmonary hemodynamics during intravenous epoprostenol (prostacyclin): A study of 15 patients with moderate to severe portopulmonary hypertension. Hepatology. 1999;30:641–648. doi: 10.1002/hep.510300307. [DOI] [PubMed] [Google Scholar]
- 19.Montani D, Savale L, Natali D, Jaïs X, Herve P, Garcia G, Humbert M, Simonneau G, Sitbon O. Long-term response to calcium-channel blockers in non-idiopathic pulmonary arterial hypertension. Eur Heart J. 2010;31:1898–1907. doi: 10.1093/eurheartj/ehq170. [DOI] [PubMed] [Google Scholar]
- 20.Victor I, Machicao VI, Fallon MB. In Clavien PA Medical care of the liver transplant patient. Wiley: Blackwell; 2012. pp. 51–61. [Google Scholar]
- 21.Krowka MJ. Hepatopulmonary syndromes. Gut. 2000;46:1–4. doi: 10.1136/gut.46.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Swanson KL, Krowka MJ. Arterial oxygenation associated with portopulmonary hypertension. Chest. 2002;121:1869–1875. doi: 10.1378/chest.121.6.1869. [DOI] [PubMed] [Google Scholar]
- 23.Galiè N, Hoeper MM, Humbert M, Torbicki A, Vachiery JL, Barbera JA, Beghetti M, Corris P, Gaine S, Gibbs JS, et al. Guidelines for the diagnosis and treatment of pulmonary hypertension: the Task Force for the Diagnosis and Treatment of Pulmonary Hypertension of the European Society of Cardiology (ESC) and the European Respiratory Society (ERS), endorsed by the International Society of Heart and Lung Transplantation (ISHLT) Eur Heart J. 2009;30:2493–2537. doi: 10.1093/eurheartj/ehp297. [DOI] [PubMed] [Google Scholar]
- 24.Ashfaq M, Chinnakotla S, Rogers L, Ausloos K, Saadeh S, Klintmalm GB, Ramsay M, Davis GL. The impact of treatment of portopulmonary hypertension on survival following liver transplantation. Am J Transplant. 2007;7:1258–1264. doi: 10.1111/j.1600-6143.2006.01701.x. [DOI] [PubMed] [Google Scholar]
- 25.Sussman N, Kaza V, Barshes N, Stribling R, Goss J, O’Mahony C, Zhang E, Vierling J, Frost A. Successful liver transplantation following medical management of portopulmonary hypertension: a single-center series. Am J Transplant. 2006;6:2177–2182. doi: 10.1111/j.1600-6143.2006.01432.x. [DOI] [PubMed] [Google Scholar]
- 26.Minder S, Fischler M, Muellhaupt B, Zalunardo MP, Jenni R, Clavien PA, Speich R. Intravenous iloprost bridging to orthotopic liver transplantation in portopulmonary hypertension. Eur Respir J. 2004;24:703–707. doi: 10.1183/09031936.04.00133203. [DOI] [PubMed] [Google Scholar]
- 27.Melgosa MT, Ricci GL, García-Pagan JC, Blanco I, Escribano P, Abraldes JG, Roca J, Bosch J, Barberà JA. Acute and long-term effects of inhaled iloprost in portopulmonary hypertension. Liver Transpl. 2010;16:348–356. doi: 10.1002/lt.21997. [DOI] [PubMed] [Google Scholar]
- 28.Raina A, Coons JC, Kanwar M, Murali S, Sokos G, Benza RL. Transitioning from parenteral treprostinil to inhaled treprostinil in patients with pulmonary arterial hypertension. Pulm Circ. 2013;3:116–120. doi: 10.4103/2045-8932.109926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hoeper MM, Seyfarth HJ, Hoeffken G, Wirtz H, Spiekerkoetter E, Pletz MW, Welte T, Halank M. Experience with inhaled iloprost and bosentan in portopulmonary hypertension. Eur Respir J. 2007;30:1096–1102. doi: 10.1183/09031936.00032407. [DOI] [PubMed] [Google Scholar]
- 30.Reichenberger F, Mainwood A, Doughty N, Fineberg A, Morrell NW, Pepke-Zaba J. Effects of nebulised iloprost on pulmonary function and gas exchange in severe pulmonary hypertension. Respir Med. 2007;101:217–222. doi: 10.1016/j.rmed.2006.05.019. [DOI] [PubMed] [Google Scholar]
- 31.Reichenberger F, Kohstall MG, Seeger T, Olschewski H, Grimminger F, Seeger W, Ghofrani HA. Effect of sildenafil on hypoxia-induced changes in pulmonary circulation and right ventricular function. Respir Physiol Neurobiol. 2007;159:196–201. doi: 10.1016/j.resp.2007.07.005. [DOI] [PubMed] [Google Scholar]
- 32.Provencher S, Souza R. Predicting survival in pulmonary arterial hypertension: time to move forward. Eur Respir J. 2010;35:958–959. doi: 10.1183/09031936.00007110. [DOI] [PubMed] [Google Scholar]
- 33.Ayoub T. Pulmonary hypertension in liver transplant. Curr Opin Organ Transplant. 2011;16:331–337. doi: 10.1097/MOT.0b013e328346e138. [DOI] [PubMed] [Google Scholar]
- 34.Wax DB, Torres A, Scher C, Leibowitz AB. Transesophageal echocardiography utilization in high-volume liver transplantation centers in the United States. J Cardiothorac Vasc Anesth. 2008;22:811–813. doi: 10.1053/j.jvca.2008.07.007. [DOI] [PubMed] [Google Scholar]
- 35.Hahn RT, Abraham T, Adams MS, Bruce CJ, Glas KE, Lang RM, Reeves ST, Shanewise JS, Siu SC, Stewart W, et al. Guidelines for performing a comprehensive transesophageal echocardiographic examination: recommendations from the American Society of Echocardiography and the Society of Cardiovascular Anesthesiologists. J Am Soc Echocardiogr. 2013;26:921–964. doi: 10.1016/j.echo.2013.07.009. [DOI] [PubMed] [Google Scholar]
- 36.Bismuth H, Castaing D, Garden OJ. Major hepatic resection under total vascular exclusion. Ann Surg. 1989;210:13–19. doi: 10.1097/00000658-198907000-00002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Gurusamy KS, Pamecha V, Davidson BR. Piggy-back graft for liver transplantation. Cochrane Database Syst Rev. 2011;(1):CD008258. doi: 10.1002/14651858.CD008258.pub2. [DOI] [PubMed] [Google Scholar]
- 38.Ramsay M. Portopulmonary hypertension and right heart failure in patients with cirrhosis. Curr Opin Anaesthesiol. 2010;23:145–150. doi: 10.1097/ACO.0b013e32833725c4. [DOI] [PubMed] [Google Scholar]
- 39.Krowka MJ. Editorial: Pulmonary hypertension, (high) risk of orthotopic liver transplantation, and some lessons from “primary” pulmonary hypertension. Liver Transpl. 2002;8:389–390. doi: 10.1053/jlts.2002.33134. [DOI] [PubMed] [Google Scholar]
- 40.Krowka MJ, Wiseman GA, Burnett OL, Spivey JR, Therneau T, Porayko MK, Wiesner RH. Hepatopulmonary syndrome: a prospective study of relationships between severity of liver disease, PaO(2) response to 100% oxygen, and brain uptake after (99m)Tc MAA lung scanning. Chest. 2000;118:615–624. doi: 10.1378/chest.118.3.615. [DOI] [PubMed] [Google Scholar]
- 41.Reich DL, Wood RK, Emre S, Bodian CA, Hossain S, Krol M, Feierman D. Association of intraoperative hypotension and pulmonary hypertension with adverse outcomes after orthotopic liver transplantation. J Cardiothorac Vasc Anesth. 2003;17:699–702. doi: 10.1053/j.jvca.2003.09.010. [DOI] [PubMed] [Google Scholar]
- 42.Machicao VI, Fallon MB. Hepatopulmonary syndrome. Semin Respir Crit Care Med. 2012;33:11–16. doi: 10.1055/s-0032-1301730. [DOI] [PubMed] [Google Scholar]
- 43.Abrams GA, Jaffe CC, Hoffer PB, Binder HJ, Fallon MB. Diagnostic utility of contrast echocardiography and lung perfusion scan in patients with hepatopulmonary syndrome. Gastroenterology. 1995;109:1283–1288. doi: 10.1016/0016-5085(95)90589-8. [DOI] [PubMed] [Google Scholar]
- 44.Schiffer E, Majno P, Mentha G, Giostra E, Burri H, Klopfenstein CE, Beaussier M, Morel P, Hadengue A, Pastor CM. Hepatopulmonary syndrome increases the postoperative mortality rate following liver transplantation: a prospective study in 90 patients. Am J Transplant. 2006;6:1430–1437. doi: 10.1111/j.1600-6143.2006.01334.x. [DOI] [PubMed] [Google Scholar]
- 45.Gómez FP, Martínez-Pallí G, Barberà JA, Roca J, Navasa M, Rodríguez-Roisin R. Gas exchange mechanism of orthodeoxia in hepatopulmonary syndrome. Hepatology. 2004;40:660–666. doi: 10.1002/hep.20358. [DOI] [PubMed] [Google Scholar]
- 46.Arguedas MR, Fallon MB. Hepatopulmonary syndrome. Clin Liver Dis. 2005;9:733–746, viii. doi: 10.1016/j.cld.2005.07.006. [DOI] [PubMed] [Google Scholar]
- 47.Saad NE, Lee DE, Waldman DL, Saad WE. Pulmonary arterial coil embolization for the management of persistent type I hepatopulmonary syndrome after liver transplantation. J Vasc Interv Radiol. 2007;18:1576–1580. doi: 10.1016/j.jvir.2007.08.008. [DOI] [PubMed] [Google Scholar]
- 48.Pastor CM, Schiffer E. Therapy Insight: hepatopulmonary syndrome and orthotopic liver transplantation. Nat Clin Pract Gastroenterol Hepatol. 2007;4:614–621. doi: 10.1038/ncpgasthep0965. [DOI] [PubMed] [Google Scholar]
- 49.Kochar R, Tanikella R, Fallon MB. Serial pulse oximetry in hepatopulmonary syndrome. Dig Dis Sci. 2011;56:1862–1868. doi: 10.1007/s10620-011-1600-7. [DOI] [PubMed] [Google Scholar]
- 50.Arguedas MR, Singh H, Faulk DK, Fallon MB. Utility of pulse oximetry screening for hepatopulmonary syndrome. Clin Gastroenterol Hepatol. 2007;5:749–754. doi: 10.1016/j.cgh.2006.12.003. [DOI] [PubMed] [Google Scholar]
- 51.Abrams GA, Sanders MK, Fallon MB. Utility of pulse oximetry in the detection of arterial hypoxemia in liver transplant candidates. Liver Transpl. 2002;8:391–396. doi: 10.1053/jlts.2002.32252. [DOI] [PubMed] [Google Scholar]
- 52.Swanson KL, Wiesner RH, Krowka MJ. Natural history of hepatopulmonary syndrome: Impact of liver transplantation. Hepatology. 2005;41:1122–1129. doi: 10.1002/hep.20658. [DOI] [PubMed] [Google Scholar]
- 53.Fallon MB, Mulligan DC, Gish RG, Krowka MJ. Model for end-stage liver disease (MELD) exception for hepatopulmonary syndrome. Liver Transpl. 2006;12:S105–S107. doi: 10.1002/lt.20971. [DOI] [PubMed] [Google Scholar]
- 54.Tanikella R, Philips GM, Faulk DK, Kawut SM, Fallon MB. Pilot study of pentoxifylline in hepatopulmonary syndrome. Liver Transpl. 2008;14:1199–1203. doi: 10.1002/lt.21482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Gupta LB, Kumar A, Jaiswal AK, Yusuf J, Mehta V, Tyagi S, Tempe DK, Sharma BC, Sarin SK. Pentoxifylline therapy for hepatopulmonary syndrome: a pilot study. Arch Intern Med. 2008;168:1820–1823. doi: 10.1001/archinte.168.16.1820. [DOI] [PubMed] [Google Scholar]
- 56.Krug S, Seyfarth HJ, Hagendorff A, Wirtz H. Inhaled iloprost for hepatopulmonary syndrome: improvement of hypoxemia. Eur J Gastroenterol Hepatol. 2007;19:1140–1143. doi: 10.1097/MEG.0b013e328220ed72. [DOI] [PubMed] [Google Scholar]
- 57.Fukushima KY, Yatsuhashi H, Kinoshita A, Ueki T, Matsumoto T, Osumi M, Matsuoka Y. Two cases of hepatopulmonary syndrome with improved liver function following long-term oxygen therapy. J Gastroenterol. 2007;42:176–180. doi: 10.1007/s00535-006-1965-0. [DOI] [PubMed] [Google Scholar]
- 58.Krowka MJ, Mandell MS, Ramsay MA, Kawut SM, Fallon MB, Manzarbeitia C, Pardo M, Marotta P, Uemoto S, Stoffel MP, et al. Hepatopulmonary syndrome and portopulmonary hypertension: a report of the multicenter liver transplant database. Liver Transpl. 2004;10:174–182. doi: 10.1002/lt.20016. [DOI] [PubMed] [Google Scholar]
- 59.Fallon MB, Krowka MJ, Brown RS, Trotter JF, Zacks S, Roberts KE, Shah VH, Kaplowitz N, Forman L, Wille K, et al. Impact of hepatopulmonary syndrome on quality of life and survival in liver transplant candidates. Gastroenterology. 2008;135:1168–1175. doi: 10.1053/j.gastro.2008.06.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Schenk P, Schöniger-Hekele M, Fuhrmann V, Madl C, Silberhumer G, Müller C. Prognostic significance of the hepatopulmonary syndrome in patients with cirrhosis. Gastroenterology. 2003;125:1042–1052. doi: 10.1016/s0016-5085(03)01207-1. [DOI] [PubMed] [Google Scholar]
- 61.Arguedas MR, Abrams GA, Krowka MJ, Fallon MB. Prospective evaluation of outcomes and predictors of mortality in patients with hepatopulmonary syndrome undergoing liver transplantation. Hepatology. 2003;37:192–197. doi: 10.1053/jhep.2003.50023. [DOI] [PubMed] [Google Scholar]
- 62.Kim JA, Lee JJ, Kim CS, Chung IS, Gwak MS, Kim GS. Does general anesthesia with inhalation anesthetics worsen hypoxemia in patients with end-stage liver disease and an intrapulmonary shunt? Transplant Proc. 2011;43:1665–1668. doi: 10.1016/j.transproceed.2011.03.056. [DOI] [PubMed] [Google Scholar]
- 63.Futier E, Constantin JM, Paugam-Burtz C, Pascal J, Eurin M, Neuschwander A, Marret E, Beaussier M, Gutton C, Lefrant JY, et al. A trial of intraoperative low-tidal-volume ventilation in abdominal surgery. N Engl J Med. 2013;369:428–437. doi: 10.1056/NEJMoa1301082. [DOI] [PubMed] [Google Scholar]
- 64.Giglio MT, Marucci M, Testini M, Brienza N. Goal-directed haemodynamic therapy and gastrointestinal complications in major surgery: a meta-analysis of randomized controlled trials. Br J Anaesth. 2009;103:637–646. doi: 10.1093/bja/aep279. [DOI] [PubMed] [Google Scholar]
- 65.Delva E, Nordlinger B, Parc R, Lienhart A, Hannoun L, Huguet C. Hepatic vascular exclusion (HVE) for major liver resections. Int Surg. 1987;72:78–81. [PubMed] [Google Scholar]
- 66.Fleming GM, Cornell TT, Welling TH, Magee JC, Annich GM. Hepatopulmonary syndrome: use of extracorporeal life support for life-threatening hypoxia following liver transplantation. Liver Transpl. 2008;14:966–970. doi: 10.1002/lt.21477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Monsel A, Mal H, Brisson H, Luo R, Eyraud D, Vézinet C, Do CH, Lu Q, Vaillant JC, Hannoun L, et al. Extracorporeal membrane oxygenation as a bridge to liver transplantation for acute respiratory distress syndrome-induced life-threatening hypoxaemia aggravated by hepatopulmonary syndrome. Crit Care. 2011;15:R234. doi: 10.1186/cc10476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Chihara Y, Egawa H, Tsuboi T, Oga T, Handa T, Yamamoto K, Mishima M, Tanaka K, Uemoto S, Chin K. Immediate noninvasive ventilation may improve mortality in patients with hepatopulmonary syndrome after liver transplantation. Liver Transpl. 2011;17:144–148. doi: 10.1002/lt.22207. [DOI] [PubMed] [Google Scholar]
- 69.Mancuso L, Scordato F, Pieri M, Valerio E, Mancuso A. Management of portopulmonary hypertension: new perspectives. World J Gastroenterol. 2013;19:8252–8257. doi: 10.3748/wjg.v19.i45.8252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Reichenberger F, Mainwood A, Morrell NW, Parameshwar J, Pepke-Zaba J. Intravenous epoprostenol versus high dose inhaled iloprost for long-term treatment of pulmonary hypertension. Pulm Pharmacol Ther. 2011;24:169–173. doi: 10.1016/j.pupt.2010.06.004. [DOI] [PubMed] [Google Scholar]
- 71.Awdish RL, Cajigas HR. Early initiation of prostacyclin in portopulmonary hypertension: 10 years of a transplant center’s experience. Lung. 2013;191:593–600. doi: 10.1007/s00408-013-9501-5. [DOI] [PubMed] [Google Scholar]
- 72.Rockey DC, Chung JJ. Reduced nitric oxide production by endothelial cells in cirrhotic rat liver: endothelial dysfunction in portal hypertension. Gastroenterology. 1998;114:344–351. doi: 10.1016/s0016-5085(98)70487-1. [DOI] [PubMed] [Google Scholar]
- 73.Gaines DI, Fallon MB. Hepatopulmonary syndrome. Liver Int. 2004;24:397–401. doi: 10.1111/j.1478-3231.2004.0944.x. [DOI] [PubMed] [Google Scholar]


