Abstract
AIM: To assess the prognostic role of baseline clinical, biochemical and radiological characteristics of patients with hepatocellular carcinoma (HCC) treated with the first transarterial chemoembolization (TACE) procedure.
METHODS: Patients with HCC treated with conventional TACE in a tertiary care setting from 1997 to 2008 were retrospectively reviewed. Predictors of survival were identified using the Cox proportional regression model.
RESULTS: Two hundred and seventy patients were included. Median age was 66 years, 81% were male, 58% were HCV-positive, 18% hepatitis B surface antigen-positive, 64% had a Child A status, 40% patients had a largest nodule diameter ≥ 5 cm and 32% had more than 3 tumor nodules. Median overall survival of the whole cohort was 25 mo (95%CI: 21.8-28.2) and the 1-, 2- and 3-year probability of survival was 80%, 50% and 31%, respectively. Non-tumor segmental portal vein thrombosis (HR = 1.76, 95%CI: 1.22-2.54), serum sodium (HR = 1.65, 95%CI: 1.25-2.18), diameter of largest nodule (HR = 1.59, 95%CI: 1.22-2.091), number of nodules (HR = 1.41, 95%CI: 1.06-1.88), alpha-fetoprotein (HR = 1.35, 95%CI: 1.03-1.76) and alkaline phosphatase (HR = 1.33, 95%CI: 1.01-1.74) were independent prognostic factors for overall survival on multivariate analysis.
CONCLUSION: The inclusion of serum sodium alongside the already known prognostic factors may allow a better prognostic definition of patients with HCC as candidates for conventional TACE.
Keywords: Liver cancer, Sorafenib, Hyponatremia, Model for end-stage liver disease sodium, Chemoembolization
Core tip: This work describes, in a retrospective cohort of 270 patients with hepatocellular carcinoma (HCC) treated with conventional transarterial chemoembolization (TACE) at our institution, the prognostic role of baseline clinical, biochemical and radiological characteristics of patients. Besides well-known prognostic parameters like portal vein thrombosis, nodule diameter and number of lesions, this study underlines the independent prognostic role of serum sodium, a well-known prognostic parameter in the field of liver cirrhosis, but less investigated in the field of hepatocellular carcinoma. Our study is the first to document the prognostic role of serum sodium in patients with HCC treated with TACE in a Western center.
INTRODUCTION
Globally, hepatocellular carcinoma (HCC) is the second most frequent cause of cancer mortality in men and the leading cause of death among patients with liver cirrhosis[1,2]. Transarterial chemoembolization (TACE) is an established treatment for patients diagnosed with HCC[3,4]. Optimal candidates for TACE are asymptomatic patients with compensated liver disease and large/multifocal HCC without extrahepatic spread or vascular invasions. However, it is also applied to patients in early stages of HCC, according to the Barcelona Clinic Liver Cancer (BCLC) classification, who are not considered for surgery or ablation or who have failed/recurred post-treatment[5]. Patients with HCC treated with TACE represent a prognostically heterogeneous population: median survival has been reported to range from 14 mo[6] to 34-48 mo[7,8] in recent series. Many studies have reported patient-, tumor- and treatment-related characteristics associated with better survival after TACE, but results from individual studies are sometimes conflicting[9,10]. Distinguishing those patients who represent good candidates for TACE from those where little or no benefit may be expected remains an unmet clinical need[11]. The aim of this study is to assess the prognostic role of baseline clinical, biochemical and radiological characteristics of patients with HCC treated with the first TACE procedure.
MATERIALS AND METHODS
Patients
Patients with HCC treated with the first TACE procedure at the Catholic University of Rome between January 1, 1997, and December 31, 2008, were retrospectively analyzed with regard to their pre-treatment characteristics. A small number of patients enrolled in this work were included in a previous paper[12]. The following data were collected for all patients before the first TACE procedure: demographic details, etiology of liver disease, biochemical data, hematological data, assessment of hepatic function based on Child-Pugh score[13], Model for End-stage Liver Disease (MELD) score and Model for End-stage Liver Disease-sodium (MELD-Na) score[14], staging of the tumor, and previous therapy. Hepatitis C and B were diagnosed by detecting antibodies to hepatitis C virus and Serum hepatitis B surface antigen (HBsAg), respectively, through standardized tests. The presence of underlying cirrhosis was assessed histologically or based on clinical and blood chemistry findings indicative of chronic liver disease together with evidence of portal hypertension (defined by the presence of at least one of the following: thrombocytopenia < 100.000/mm3, gastro-esophageal varices or splenomegaly). Extra-hepatic disease was assessed with abdominal multiphasic Computed Tomography (CT) or Magnetic Resonance Imaging and chest radiography. Portal vein thrombosis was classified as non-tumor on the basis of the lack of contrast enhancement in the arterial phase. Bone metastases were sought using scintigraphy, if clinically suspected. Follow-up ended on September 30, 2012. The date of death was determined by telephone interview if more than 3 mo had elapsed since the last follow-up visit and death did not occur in our hospital or was not reported by the family. Patients underwent Orthotopic Liver Transplantation (OLT) after TACE were excluded from the analysis.
TACE procedure
The indications for conventional TACE at our institution were: (1) HCC diagnosed by pathology or by non-invasive criteria, according to Barcelona criteria until 2005[15], and subsequently according to AASLD guidelines[16]; (2) patients with HCC that were not candidates for resection or ablation or had failed/recurred after resection/ablation; (3) adequate hepatic function (Child-Pugh score 5-7 points); and (4) performance status (PS) = 0[17].
Absolute and relative contraindications were: (1) hypovascular HCC; (2) infiltrative HCC; (3) presence of vascular invasion or extrahepatic metastases; (4) widespread HCC (defined as involving > 50% of the liver); (5) portal vein thrombosis (partial or complete) of the trunk or the right or left portal branch or hepatofugal blood flow[9]; (6) presence of a Transjugular Intrahepatic Portosystemic Shunt[9]; (7) esophageal varices F2 with red signs or F3 untreated[9]; (8) inadequate hepatic function (albumin < 2.8 g/dL, bilirubin > 3 mg/dL and alanine and aspartate aminotransferase > 5 times the upper limit of the normal range); (9) clinically detectable ascites (grade 2-3)[18]; (10) renal insufficiency (creatinine ≥ 2 mg/dL or creatinine clearance < 30 mL/min); (11) inadequate clotting profile (platelet count < 40 × 109/L, hemoglobin < 8.5 g/dL, and International Normalized Ratio > 1.5); (12) contraindication for iodinated contrast media; and (13) contraindication for arterial endovascular procedure.
TACE was performed by using selective catheterization of the hepatic segmental arteries feeding the lesions. A mixture of lipiodol (Lipiodol Ultrafluid, Mitsui, Tokyo, Japan) and carboplatin 450 mg was injected, followed by selective arterial embolization by using gelatin sponge particles (Spongostan Standard; Johnson and Johnson Medical, Gargrave, Skipton, England). One month after TACE, patients underwent a multiphasic CT scan. TACE was repeated on demand, at least 2 mo after the first procedure, in patients with evidence of viable tumor persistence, as defined by the amended RECIST criteria[15]. TACE was withheld or discontinued whenever vascular contraindications, poor hepatic function, severe adverse effects, progressive disease with vascular involvement or extrahepatic metastases developed. No treatment with Drug-Eluting Beads (DEB) was included in this cohort.
Statistical analysis
Continuous data were expressed as the median ± interquartile range. Overall survival was calculated using the Kaplan-Meier function and expressed as median and 95% confidence interval. A univariate analysis to identify baseline demographic, clinical, biochemical and radiological predictors of survival at the time of the first TACE procedure was performed using the Kaplan-Meier method of survival function. For continuous variables, median values were used to determine the cut-off. Variables with an alpha less than 0.25 at the univariate analysis were included in a backward Cox proportional regression model to identify independent predictors of survival.
Patients were classified according to five prognostic staging systems for HCC: Okuda staging system[19], Cancer of the Liver Italian Program score[20], BCLC staging system[21], Tokyo score[22], Japanese Integrated System (JIS)[23]. For these scores the Area under the curve was calculated and the comparisons between curves was performed according to the method developed by Hanley and McNeil[24]. Moreover, for each score, survival curves calculated by the Kaplan-Meier method were compared using the log rank test.
All statistical analyses were performed using SPSS version 19.0 (SPSS Inc., Chicago, IL).
RESULTS
Patient characteristics
A total of 270 patients with HCC were treated with the first TACE procedure during the abovementioned time period. Table 1 shows the demographic, clinical, and tumor information for all patients. The majority of the patients were men (81%); the median age was 66 years. Almost all (91%) met criteria of having cirrhosis, the most common cause being Hepatitis C Virus (58%). One hundred and seventy one (64%) patients were Child-Pugh class A; the median MELD and MELD-Na scores were 9 and 10, respectively. Forty-one (15%) had non-tumor segmental portal vein thrombosis. One hundred and eight (40%) patients had a largest nodule diameter ≥ 5 cm and 86 (32%) had more than 3 tumor nodules. One hundred and ninety-seven (73%) patients had never been treated, 53 (20%) had been previously treated with percutaneous ablation and 24 (9%) with surgical resection. Median Alpha-Fetoprotein (AFP) level was 24 ng/mL, median serum sodium level was 138 mEq/L and median alkaline phosphatase level was 261 UI/L.
Table 1.
Baseline predictors of survival in 270 patients with hepatocellular carcinoma at time of first conventional transarterial chemoembolization n (%)
| Variables | n | Patients or median ± IQR | Univariate analysis |
Multivariate analysis |
||
| HR (95%CI) | P value | HR (95%CI) | P value | |||
| Patient-related characteristics | ||||||
| Age (yr) | 270 | 66 ± 13 | - | NS | - | - |
| Male sex | 270 | 220 (81.5) | - | NS | - | - |
| Diabetes | 270 | 61 (22.6) | - | NS | - | - |
| Etiology | ||||||
| Hepatitis C Virus | 270 | 158 (58.5) | - | NS | - | - |
| Hepatitis B Virus | 270 | 50 (18.5) | 0.685 (0.484-0.968) | 0.032 | - | NS |
| Alcohol | 270 | 13 (4.8) | - | NS | - | - |
| Other/unknown | 270 | 49 (18.2) | - | NS | - | - |
| Liver cirrhosis | 270 | 246 (91.1) | - | NS | - | - |
| Child-Pugh class A | 268 | 171 (63.8) | - | NS | - | - |
| MELD | 270 | 9 ± 4 | - | NS | - | - |
| MELD-Na | 270 | 10 ± 3 | - | NS | - | - |
| Ascites (radiological only) | 270 | 61 (22.6) | - | NS | - | - |
| Encephalopathy | 269 | 18 (6.7) | - | NS | - | - |
| Esophageal varices | 270 | 134 (49.6) | - | NS | - | - |
| Segmental portal vein thrombosis (non-tumor) | 270 | 41 (15.2) | 1.708 (1.203-2.424) | 0.003 | 1.764 (1.224-2.542) | 0.001 |
| Tumor burden | ||||||
| Number of nodules > 3 | 270 | 86 (31.8) | 1.622 (1.232-2.136) | 0.001 | 1.41 (1.060-1.876) | 0.018 |
| Diameter of largest nodule ≥ 5 cm | 269 | 108 (40.2) | 1.557 (1.198-2.022) | 0.001 | 1.595 (1.217-2.091) | 0.001 |
| Bilobarity | 264 | 132 (48.9) | - | NS | - | - |
| Previous therapy1 | 270 | 73 (27) | - | NS | - | - |
| Percutaneous Ethanol Injection | 270 | 45 (16.7) | - | NS | - | - |
| Radiofrequency ablation | 270 | 8 (3) | - | NS | - | - |
| Liver resection | 270 | 24 (8.9) | - | NS | - | - |
| Baseline laboratory values | ||||||
| Alanine aminotransferase (UI/L) | 270 | 55 ± 63.5 | - | NS | - | - |
| Gamma-glutamyl transferase (UI/L) | 269 | 71 ± 80 | - | NS | - | - |
| Alkaline phosphatase (UI/L) | 269 | 261 ± 175 | 1.542 (1.188-2.002) | 0.001 | 1.326 (1.008-1.743) | 0.043 |
| Albumin (g/dL) | 269 | 3.5 ± 0.8 | - | NS | - | - |
| Serum sodium (mEq/L) | 270 | 138 ± 5 | 1.527 (1.168-1.995) | 0.002 | 1.648 (1.249-2.176) | 0.000 |
| International normalised ratio | 270 | 1.13 ± 0.19 | - | NS | - | - |
| Total bilirubin (mg/dL) | 270 | 1.1 ± 0.6 | - | NS | - | - |
| Creatinine (mg/dL) | 270 | 1 ± 0.3 | - | NS | - | - |
| Alpha-fetoprotein (ng/mL) | 267 | 24 ± 328 | 1.428 (1.099-1.856) | 0.008 | 1.346 (1.027-1.763) | 0.031 |
Some patients were subjected to more than one method of treatment before chemoembolization. Univariate analysis by Kaplan-Meier method of survival function. Multivariate analysis by backward stepwise cox regression. For continuous variables, median values were used to determine the cut-off. NS: Not significant; MELD: Model for End-stage Liver Disease; MELD-Na: MELD-sodium.
Survival
At the time the data were analyzed, 232 (86%) patients had died. Of the 38 not known to have died, 14 were lost to follow-up after a median of 11.0 mo (95%CI: 3.7-18.3), and the remaining 24 were censored at the end of our follow-up period at 9/30/2012 with a median follow-up time of 48.0 mo (95%CI: 42.0-54.0). The overall median survival of the entire cohort was 25 mo (95%CI 21.8-28.2 mo, Figure 1A) and the 1-, 2- and 3-year survival probability was 80%, 50% and 31% respectively.
Figure 1.
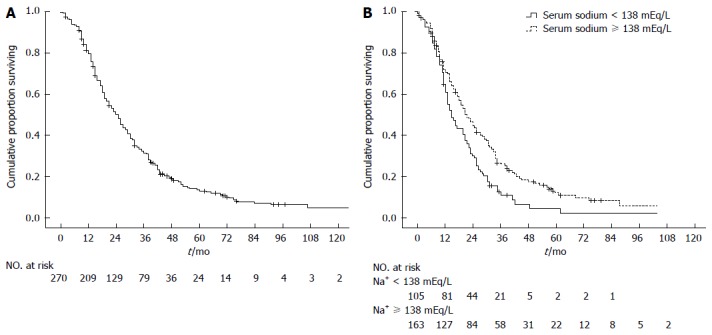
Kaplan-Meier analysis of overall survival. A: Among 270 patients, the median overall survival was 25 mo (95%CI: 21.8-28.2); B: According to baseline serum sodium (105 patients with serum sodium < 138 mEq/L and 163 patients with serum sodium ≥ 138 mEq/L): median overall survival was respectively 19 mo (95%CI: 15.4-22.1) and 26 mo (95%CI: 21.8-30.2); hazard ratio for death in the serum sodium < 138 mEq/L group 1.65 (95%CI: 1.25-2.18).
Baseline predictors of survival
Univariate analysis showed that non-tumor segmental portal vein thrombosis, number of nodules, largest nodule diameter, alkaline phosphatase, serum sodium, AFP and HBsAg positivity were significant baseline predictors of survival in HCC patients treated with TACE (Table 1). Serum sodium was a significant prognostic factor even when each continuous variable was put individually into a univariate Cox regression model: < 138 mEq/L (i.e., the median value) was the best cut-off (P = 0.002, Figure 1B), although < 135 mEq/L was still significant (P = 0.022). Cox regression analysis identified non-tumor segmental portal vein thrombosis (P = 0.001), serum sodium (P < 0.001), largest nodule diameter (P = 0.001), number of nodules (P = 0.018), AFP (P = 0.031), and alkaline phosphatase (P = 0.043) as independent baseline predictors of survival for the entire cohort of HCC patients treated with TACE (Table 1). Since serum sodium concentration can be influenced by concomitant treatment with diuretics, we repeated statistical analysis excluding patients with ascites (n = 61), which would have had an indication for diuretic therapy. Univariate and multivariate analysis again confirmed the role of serum sodium as an independent predictor of survival (P = 0.002), even excluding patients with ascites.
Staging systems
Table 2 describes the distribution of patients across five prognostic staging systems for HCC (Okuda, CLIP, BCLC, Tokyo, JIS) with respective median survivals for each stage. When the five staging systems were analyzed separately using Kaplan-Meier survival analysis (n = 270), each staging system showed a significant difference in the probability of survival across the different stages with the exception of the Okuda system. Univariate analysis for staging systems, performed through Cox backward stepwise regression, showed that CLIP (HR = 1.73, 95%CI: 1.06-2.82; P = 0.027), BCLC (HR = 1.80, 95%CI: 1.24-2.60; P = 0.002), Tokyo (HR = 1.55, 95%CI: 1.19-2.01; P = 0.001) and JIS (HR = 1.78, 95%CI: 1.26-2.51; P = 0.001) were significant baseline predictors of survival in HCC patients treated with TACE. When staging systems were studied in multivariate analysis along with the individual variables (i.e., clinical, radiological and laboratory parameters), none of them was statistically significant. In Table 3 the comparison of ROC curves is shown demonstrating that significant differences between curves exist between CLIP or JIS vs OKUDA scores for the survival outcome at 1 year.
Table 2.
Survival according to five staging systems in 270 hepatocellular carcinoma patients treated with conventional transarterial chemoembolization n (%)
| Staging system | Median survival (95%CI)-mo | P value | |
| Okuda | NS | ||
| I | 161 (59.6) | 27.00 (23.02-30.98) | |
| II | 103 (38.1) | 21.00 (17.94-24.06) | |
| III | 6 (2.2) | 16.00 (5.20-26.80) | |
| CLIP | 0.001 | ||
| 0 | 45 (16.7) | 32.00 (23.18-40.82) | |
| 1 | 90 (33.3) | 30.00 (21.02-38.98) | |
| 2 | 70 (25.9) | 26.00 (24.23-27.77) | |
| 3 | 46 (17) | 15.00 (10.57-19.43) | |
| 4 | 19 (17) | 13.00 (5.89-20.11) | |
| BCLC | 0.013 | ||
| A | 87 (32.2) | 30.00 (24.07-35.93) | |
| B | 153 (56.7) | 22.00 (17.11-24.89) | |
| C1 | 30 (11.1) | 19.00 (6.92-31.08) | |
| Tokyo | 0.011 | ||
| 0 | 3 (1.1) | 26.00 (-) | |
| 1 | 24 (8.9) | 29.00 (9.65-48.35) | |
| 2 | 44 (16.3) | 30.00 (15.88-44.12) | |
| 3 | 52 (19.3) | 32.00 (27.45-36.55) | |
| 4 | 147 (54.4) | 20.00 (17.12-22.88) | |
| JIS | 0.003 | ||
| 0 | 3 (1.1) | 26.00 (-) | |
| 1 | 104 (38.5) | 29.00 (22.83-35.17) | |
| 2 | 121 (44.8) | 25.00 (19.38-30.62) | |
| 3 | 42 (15.6) | 17.00 (13.46-20.54) | |
Included 25 patients with extrahepatic metastases and Child A status and 5 patients with a performance status of 1 or 2 and Child B8-9; the decision to perform transarterial chemoembolization in these patients was made on an individual basis in an era when sorafenib was not available. For each score, survival curves calculated by the Kaplan-Meier method were compared using the log rank test. A dash (-) indicates that the CI could not be calculated if the number of patients within each prognostic score was ≤ 5. NS: Not significant; CLIP: Cancer of the Liver Italian Program; BCLC: Barcelona Clinic Liver Cancer; JIS: Japanese Integrated System.
Table 3.
Comparisons between receiver operating characteristic curves n (%)
| Staging system | AUROC 1 yr | AUROC 2 yr |
| (95%CI) [P value] | (95%CI) (P value) | |
| OKUDA | 0.492 (0.410-0.574) [0.849] | 0.547 (0.478-0.615) [0.185] |
| CLIP | 0.632 (0.548-0.716) [0.002]1 | 0.603 (0.536-0.670) [0.003] |
| BCLC | 0.600 (0.524-0.676) [0.018] | 0.571 (0.502-0.639) [0.045] |
| TOKYO | 0.541 (0.457-0.626) [0.324] | 0.582 (0.514-0.650) [0.020] |
| JIS | 0.658 (0.582-0.734) [< 0.001]2 | 0.576 (0.508-0.644) [0.031] |
Significant differences between curves:
CLIP vs OKUDA;
JIS vs OKUDA. AUROC: Area under the curve; CLIP: Cancer of the Liver Italian Program; BCLC: Barcelona Clinic Liver Cancer; JIS: Japanese Integrated System.
DISCUSSION
In our cohort of 270 HCC patients treated with conventional TACE, non-tumor segmental portal vein thrombosis, serum sodium, largest nodule diameter, number of nodules, AFP, and alkaline phosphatase were independent baseline predictors of survival.
Negative baseline prognostic factors for TACE already described in literature[9] include both non-tumor portal vein thrombosis and portal tumor invasion, the first because of the lack of portal blood flow and resulting risk of ischemic necrosis of viable liver, the second because it is a well-defined unfavorable prognostic factor for HCC. In our study, in patients with non-tumor segmental portal vein thrombosis, an individual decision was made for or against TACE on the basis of liver function and respective location of tumor nodules and segmental thrombosis. Nevertheless, non-tumor segmental portal vein thrombosis remains an important risk factor for death, with the highest hazard ratio in our cohort. While Asian guidelines still recommend TACE for these patients[25], recently a panel of experts proposed to classify patients with segmental thrombosis and Child-Pugh class A, as "quasi-C" and to treat them directly with sorafenib or alternatively with transarterial radioembolization or TACE[26]. Studies comparing endovascular or systemic treatments, or combination of these, in patients with HCC and portal vein thrombosis are ongoing.
The peculiar result of our study is the evidence of the prognostic role of serum sodium in HCC patients treated by TACE. Hyponatremia is a frequent complication of advanced cirrhosis related to arterial splanchnic vasodilatation, which involves a reduction of effective circulating volume. The circulatory dysfunction causes a non osmotic hypersecretion of arginine vasopressin (or antidiuretic hormone) from the neurohypophysis, which causes retention of water that is disproportionate to the retention of sodium, thus resulting in hypervolemic hyponatremia and hypo-osmolality[27]. Serum sodium may be a good indicator of the intensity of this pathophysiological disturbance and has been recognized as an important prognostic factor in patients with liver cirrhosis. Hyponatremia has been associated with ascites[28,29], hepatorenal syndrome[29-31], bacterial infections[28,29], and hepatic encephalopathy[29,32]. Finally, hyponatremia has also been associated with death from liver disease[28,30,33] and serum sodium was included in the score used to predict short-term mortality in patients on the waiting list for OLT[14].
The prognostic role of serum sodium has been less investigated in the field of HCC. A retrospective study performed in Italy on 466 patients undergoing hepatectomy for HCC on cirrhosis identified serum sodium as a risk factor for irreversible postoperative liver failure in patients with MELD score 9-10[34]. Serum sodium was an independent risk factor for mortality in a cohort of 116 HCC patients consecutively evaluated for OLT[35]. In a large cohort of unselected HCC patients from Taiwan, MELD-Na was a better predictor of 3-mo and 6-mo survival than MELD[36]; in the opinion of the investigators hyponatremia may be more sensitive than serum creatinine in detecting the subtle but critical deterioration of renal function in HCC patients with liver cirrhosis. Accordingly, an intriguing explanation of the prognostic role of serum sodium in patients with HCC treated with TACE hypothesized that serum sodium was potentially able to identify patients at greater risk of renal failure induced by radiocontrast agents. A prospective study conducted in Taiwan on 591 patients with HCC treated with TACE has refuted this hypothesis, while confirming the independent prognostic role of serum sodium[37]. In a recent study from South Korea, serum sodium is recognized as one of the risk factors for acute hepatic failure after TACE[38]. Our study is the first to document the prognostic role of serum sodium in patients with HCC treated with TACE in a Western center.
In our study, the Cox regression model identified < 138 mEq/L as the best cut-off for serum sodium, although the cut-off of < 135 mEq/L was still significant. At first glance, interpreting serum sodium values that fall within the normal range as a risk factor for mortality may seem disconcerting, but this has already been described in literature[34,36-38], although the exact value of serum sodium at which the risk of mortality arises remains to be identified. In contrast to serum sodium, the MELD-Na was not a significant prognostic factor in our study, probably because 80% of patients were in the range 8-14.
Beside well-known prognostic factors such as largest nodule diameter ≥ 5 cm, presence of > 3 nodules and elevated AFP[9], our study shows that alkaline phosphatase was an independent baseline predictor of survival in patients with HCC treated with conventional TACE. The association between an elevation of alkaline phosphatase and a poor prognosis in patients with HCC is not completely understood in literature, although alkaline phosphatase has been found an independent predictor of both disease-free and overall survival after hepatectomy[39,40] and is included in some staging systems for HCC like the Chinese University Prognostic Index[41] and Group d’Etude de Traitement du Carcinoma Hepatocellulaire[42]. A relationship with biliary infiltration seems unlikely[39]. Some authors suggest that alkaline phosphatase, a mesenchymal stem cell antigen with nucleolar localization in Hep-G2 cancer cells, could be a marker of cancer proliferation[39], while others speculate that elevation of alkaline phosphatase is a marker of inflammatory necrosis in the liver, which was associated with an increased risk of recurrence after hepatectomy[40].
Similar to other studies[43], our study has confirmed that the most commonly used staging systems (BCLC, CLIP, Tokyo, JIS) have capacity to discriminate prognosis in patients with HCC treated with conventional TACE, but none of them turned out to be higher than individual variables from which it is composed.
The limitations of this study include its retrospective nature and incomplete knowledge of the clinical history of patients from the time of TACE to death, especially with regard to time to progression, the onset of renal or hepatic decompensation, and treatment performed after first TACE (e.g., number of repeated TACE or percutaneous ablation performed after TACE). The high number of cases (86%) for which data on patient survival is available strengthens the findings of our analysis.
Given the prognostic role of serum sodium in patients with HCC treated with TACE, it would be interesting to investigate its prognostic role in cohorts of HCC patients treated with DEB-TACE, sorafenib or radioembolization. The inclusion of serum sodium alongside the already known prognostic factors may allow a better prognostic definition of patients with HCC as candidates for conventional TACE.
COMMENTS
Background
Transarterial chemoembolization is an established treatment for patients diagnosed with hepatocellular carcinoma with compensated liver disease and not suitable for surgery or ablation. These patients represent a prognostically heterogeneous population, with median survival ranging from 14 to 48 mo. Distinguishing those patients who represent good candidates for transarterial chemoembolization from those where little or no benefit may be expected remains an unmet clinical need.
Research frontiers
Many studies have reported patient-, tumor- and treatment-related characteristics associated with better survival after transarterial chemoembolization for hepatocellular carcinoma, but results from individual studies are sometimes conflicting. An important research hotspot in this area is to distinguish patients who will benefit from transarterial chemoembolization compared to other treatment options (such as radioembolization and sorafenib).
Innovations and breakthroughs
The peculiar result of our study is the evidence of the prognostic role of serum sodium in patients with hepatocellular carcinoma treated by transarterial chemoembolization. Serum sodium has been already recognized as an important prognostic factor in patients with liver cirrhosis and has been associated with ascites, hepatorenal syndrome, bacterial infections, hepatic encephalopathy and death from liver disease, but its prognostic role in the field of hepatocellular carcinoma has been less investigated. Two studies identified serum sodium as an independent risk factor for mortality in patients with hepatocellular carcinoma underwent hepatectomy and liver transplantation, respectively. Two studies performed in Taiwan and South Korea, respectively, described the independent prognostic role of serum sodium in patients with hepatocellular carcinoma treated by transarterial chemoembolization. The authors must remember that in the eastern countries the characteristics of patients with hepatocellular carcinoma and the transarterial chemoembolization procedures are different than in Western countries. The authors’ study is the first to document the prognostic role of serum sodium in patients with hepatocellular carcinoma treated with transarterial chemoembolization in a Western center.
Applications
The study results suggest that serum sodium is a readily available, objective, and reproducible prognostic factor in patients with hepatocellular carcinoma candidates for conventional transarterial chemoembolization procedure. Future perspectives include investigating its prognostic role in patients with hepatocellular carcinoma treated with drug-eluting beads transarterial chemoembolization, sorafenib or radioembolization.
Terminology
Hepatocellular carcinoma is a malignant tumor of the liver and represents the leading cause of death among patients with liver cirrhosis. Transarterial chemoembolization is a radiological procedure for the treatment of hepatocellular carcinoma which consists in administering a mixture composed of chemotherapeutic agents and embolizing substances directly into the malignant hepatic nodule using an endovascular catheter.
Peer review
The authors retrospectively assess the outcomes of 270 patients undergoing conventional transarterial chemoembolization over an 11 year timeframe to determine variables associated with overall survival. The clinical and demographic characteristics were representative of a western population undergoing transarterial chemoembolization. Median overall survival was 25 mo, which seems reasonable. Expected parameters associated with overall survival were confirmed to be associated (non-malignant portal venous thrombosis, tumor number, tour size, alpha-fetoprotein). In multivariate analysis serum sodium < 138 mEq/L but not albumin and bilirubin was associated with overall survival. This paper addressed the question thoroughly. All methods were sound. Authors did not overreach with the data analysis. Very sound paper with valid conclusions.
Footnotes
P- Reviewers: Di Donato R, Kaplan DE, Kanda T, Sazci A S- Editor: Ma YJ L- Editor: A E- Editor: Zhang DN
References
- 1.Jemal A, Bray F, Center MM, Ferlay J, Ward E, Forman D. Global cancer statistics. CA Cancer J Clin. 2011;61:69–90. doi: 10.3322/caac.20107. [DOI] [PubMed] [Google Scholar]
- 2.Alazawi W, Cunningham M, Dearden J, Foster GR. Systematic review: outcome of compensated cirrhosis due to chronic hepatitis C infection. Aliment Pharmacol Ther. 2010;32:344–355. doi: 10.1111/j.1365-2036.2010.04370.x. [DOI] [PubMed] [Google Scholar]
- 3.Bruix J, Sherman M. Management of hepatocellular carcinoma: an update. Hepatology. 2011;53:1020–1022. doi: 10.1002/hep.24199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Biolato M, Marrone G, Racco S, Di Stasi C, Miele L, Gasbarrini G, Landolfi R, Grieco A. Transarterial chemoembolization (TACE) for unresectable HCC: a new life begins? Eur Rev Med Pharmacol Sci. 2010;14:356–362. [PubMed] [Google Scholar]
- 5.European Association For The Study Of The Liver; European Organisation For Research And Treatment Of Cancer. EASL-EORTC clinical practice guidelines: management of hepatocellular carcinoma. J Hepatol. 2012;56:908–943. doi: 10.1016/j.jhep.2011.12.001. [DOI] [PubMed] [Google Scholar]
- 6.Doffoël M, Bonnetain F, Bouché O, Vetter D, Abergel A, Fratté S, Grangé JD, Stremsdoerfer N, Blanchi A, Bronowicki JP, et al. Multicentre randomised phase III trial comparing Tamoxifen alone or with Transarterial Lipiodol Chemoembolisation for unresectable hepatocellular carcinoma in cirrhotic patients (Fédération Francophone de Cancérologie Digestive 9402) Eur J Cancer. 2008;44:528–538. doi: 10.1016/j.ejca.2008.01.004. [DOI] [PubMed] [Google Scholar]
- 7.Takayasu K, Arii S, Ikai I, Omata M, Okita K, Ichida T, Matsuyama Y, Nakanuma Y, Kojiro M, Makuuchi M, et al. Prospective cohort study of transarterial chemoembolization for unresectable hepatocellular carcinoma in 8510 patients. Gastroenterology. 2006;131:461–469. doi: 10.1053/j.gastro.2006.05.021. [DOI] [PubMed] [Google Scholar]
- 8.Burrel M, Reig M, Forner A, Barrufet M, de Lope CR, Tremosini S, Ayuso C, Llovet JM, Real MI, Bruix J. Survival of patients with hepatocellular carcinoma treated by transarterial chemoembolisation (TACE) using Drug Eluting Beads. Implications for clinical practice and trial design. J Hepatol. 2012;56:1330–1335. doi: 10.1016/j.jhep.2012.01.008. [DOI] [PubMed] [Google Scholar]
- 9.Raoul JL, Sangro B, Forner A, Mazzaferro V, Piscaglia F, Bolondi L, Lencioni R. Evolving strategies for the management of intermediate-stage hepatocellular carcinoma: available evidence and expert opinion on the use of transarterial chemoembolization. Cancer Treat Rev. 2011;37:212–220. doi: 10.1016/j.ctrv.2010.07.006. [DOI] [PubMed] [Google Scholar]
- 10.Grieco A, Marcoccia S, Miele L, Marmiroli L, Caminiti G, Ragazzoni E, Cotroneo AR, Cefaro GA, Rapaccini GL, Gasbarrini G. Transarterial chemoembolization (TACE) for unresectable hepatocellular carcinoma in cirrhotics: functional hepatic reserve and survival. Hepatogastroenterology. 2003;50:207–212. [PubMed] [Google Scholar]
- 11.Farinati F, Giacomin A, Vanin V, Giannini E, Trevisani F. TACE treatment in hepatocellular carcinoma: what should we do now? J Hepatol. 2012;57:221–222. doi: 10.1016/j.jhep.2011.12.022. [DOI] [PubMed] [Google Scholar]
- 12.Grieco A, Pompili M, Caminiti G, Miele L, Covino M, Alfei B, Rapaccini GL, Gasbarrini G. Prognostic factors for survival in patients with early-intermediate hepatocellular carcinoma undergoing non-surgical therapy: comparison of Okuda, CLIP, and BCLC staging systems in a single Italian centre. Gut. 2005;54:411–418. doi: 10.1136/gut.2004.048124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Pugh RN, Murray-Lyon IM, Dawson JL, Pietroni MC, Williams R. Transection of the oesophagus for bleeding oesophageal varices. Br J Surg. 1973;60:646–649. doi: 10.1002/bjs.1800600817. [DOI] [PubMed] [Google Scholar]
- 14.Kim WR, Biggins SW, Kremers WK, Wiesner RH, Kamath PS, Benson JT, Edwards E, Therneau TM. Hyponatremia and mortality among patients on the liver-transplant waiting list. N Engl J Med. 2008;359:1018–1026. doi: 10.1056/NEJMoa0801209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bruix J, Sherman M, Llovet JM, Beaugrand M, Lencioni R, Burroughs AK, Christensen E, Pagliaro L, Colombo M, Rodés J. Clinical management of hepatocellular carcinoma. Conclusions of the Barcelona-2000 EASL conference. European Association for the Study of the Liver. J Hepatol. 2001;35:421–430. doi: 10.1016/s0168-8278(01)00130-1. [DOI] [PubMed] [Google Scholar]
- 16.Bruix J, Sherman M. Management of hepatocellular carcinoma. Hepatology. 2005;42:1208–1236. doi: 10.1002/hep.20933. [DOI] [PubMed] [Google Scholar]
- 17.Sørensen JB, Klee M, Palshof T, Hansen HH. Performance status assessment in cancer patients. An inter-observer variability study. Br J Cancer. 1993;67:773–775. doi: 10.1038/bjc.1993.140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.European Association for the Study of the Liver. EASL clinical practice guidelines on the management of ascites, spontaneous bacterial peritonitis, and hepatorenal syndrome in cirrhosis. J Hepatol. 2010;53:397–417. doi: 10.1016/j.jhep.2010.05.004. [DOI] [PubMed] [Google Scholar]
- 19.Okuda K, Ohtsuki T, Obata H, Tomimatsu M, Okazaki N, Hasegawa H, Nakajima Y, Ohnishi K. Natural history of hepatocellular carcinoma and prognosis in relation to treatment. Study of 850 patients. Cancer. 1985;56:918–928. doi: 10.1002/1097-0142(19850815)56:4<918::aid-cncr2820560437>3.0.co;2-e. [DOI] [PubMed] [Google Scholar]
- 20.Prospective validation of the CLIP score: a new prognostic system for patients with cirrhosis and hepatocellular carcinoma. The Cancer of the Liver Italian Program (CLIP) Investigators. Hepatology. 2000;31:840–845. doi: 10.1053/he.2000.5628. [DOI] [PubMed] [Google Scholar]
- 21.Llovet JM, Brú C, Bruix J. Prognosis of hepatocellular carcinoma: the BCLC staging classification. Semin Liver Dis. 1999;19:329–338. doi: 10.1055/s-2007-1007122. [DOI] [PubMed] [Google Scholar]
- 22.Tateishi R, Yoshida H, Shiina S, Imamura H, Hasegawa K, Teratani T, Obi S, Sato S, Koike Y, Fujishima T, et al. Proposal of a new prognostic model for hepatocellular carcinoma: an analysis of 403 patients. Gut. 2005;54:419–425. doi: 10.1136/gut.2003.035055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kudo M, Chung H, Haji S, Osaki Y, Oka H, Seki T, Kasugai H, Sasaki Y, Matsunaga T. Validation of a new prognostic staging system for hepatocellular carcinoma: the JIS score compared with the CLIP score. Hepatology. 2004;40:1396–1405. doi: 10.1002/hep.20486. [DOI] [PubMed] [Google Scholar]
- 24.Hanley JA, McNeil BJ. A method of comparing the areas under receiver operating characteristic curves derived from the same cases. Radiology. 1983;148:839–843. doi: 10.1148/radiology.148.3.6878708. [DOI] [PubMed] [Google Scholar]
- 25.Omata M, Lesmana LA, Tateishi R, Chen PJ, Lin SM, Yoshida H, Kudo M, Lee JM, Choi BI, Poon RT, et al. Asian Pacific Association for the Study of the Liver consensus recommendations on hepatocellular carcinoma. Hepatol Int. 2010;4:439–474. doi: 10.1007/s12072-010-9165-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bolondi L, Burroughs A, Dufour JF, Galle PR, Mazzaferro V, Piscaglia F, Raoul JL, Sangro B. Heterogeneity of patients with intermediate (BCLC B) Hepatocellular Carcinoma: proposal for a subclassification to facilitate treatment decisions. Semin Liver Dis. 2012;32:348–359. doi: 10.1055/s-0032-1329906. [DOI] [PubMed] [Google Scholar]
- 27.Ginès P, Guevara M. Hyponatremia in cirrhosis: pathogenesis, clinical significance, and management. Hepatology. 2008;48:1002–1010. doi: 10.1002/hep.22418. [DOI] [PubMed] [Google Scholar]
- 28.Borroni G, Maggi A, Sangiovanni A, Cazzaniga M, Salerno F. Clinical relevance of hyponatraemia for the hospital outcome of cirrhotic patients. Dig Liver Dis. 2000;32:605–610. doi: 10.1016/s1590-8658(00)80844-0. [DOI] [PubMed] [Google Scholar]
- 29.Angeli P, Wong F, Watson H, Ginès P. Hyponatremia in cirrhosis: Results of a patient population survey. Hepatology. 2006;44:1535–1542. doi: 10.1002/hep.21412. [DOI] [PubMed] [Google Scholar]
- 30.Ginès A, Escorsell A, Ginès P, Saló J, Jiménez W, Inglada L, Navasa M, Clària J, Rimola A, Arroyo V. Incidence, predictive factors, and prognosis of the hepatorenal syndrome in cirrhosis with ascites. Gastroenterology. 1993;105:229–236. doi: 10.1016/0016-5085(93)90031-7. [DOI] [PubMed] [Google Scholar]
- 31.Porcel A, Díaz F, Rendón P, Macías M, Martín-Herrera L, Girón-González JA. Dilutional hyponatremia in patients with cirrhosis and ascites. Arch Intern Med. 2002;162:323–328. doi: 10.1001/archinte.162.3.323. [DOI] [PubMed] [Google Scholar]
- 32.Guevara M, Baccaro ME, Torre A, Gómez-Ansón B, Ríos J, Torres F, Rami L, Monté-Rubio GC, Martín-Llahí M, Arroyo V, et al. Hyponatremia is a risk factor of hepatic encephalopathy in patients with cirrhosis: a prospective study with time-dependent analysis. Am J Gastroenterol. 2009;104:1382–1389. doi: 10.1038/ajg.2009.293. [DOI] [PubMed] [Google Scholar]
- 33.Jenq CC, Tsai MH, Tian YC, Chang MY, Lin CY, Lien JM, Chen YC, Fang JT, Chen PC, Yang CW. Serum sodium predicts prognosis in critically ill cirrhotic patients. J Clin Gastroenterol. 2010;44:220–226. doi: 10.1097/MCG.0b013e3181aabbcd. [DOI] [PubMed] [Google Scholar]
- 34.Cescon M, Cucchetti A, Grazi GL, Ferrero A, Viganò L, Ercolani G, Zanello M, Ravaioli M, Capussotti L, Pinna AD. Indication of the extent of hepatectomy for hepatocellular carcinoma on cirrhosis by a simple algorithm based on preoperative variables. Arch Surg. 2009;144:57–63; discussion 63. doi: 10.1001/archsurg.2008.522. [DOI] [PubMed] [Google Scholar]
- 35.Meza-Junco J, Montano-Loza AJ, Baracos VE, Prado CM, Bain VG, Beaumont C, Esfandiari N, Lieffers JR, Sawyer MB. Sarcopenia as a prognostic index of nutritional status in concurrent cirrhosis and hepatocellular carcinoma. J Clin Gastroenterol. 2013;47:861–870. doi: 10.1097/MCG.0b013e318293a825. [DOI] [PubMed] [Google Scholar]
- 36.Hsu CY, Huang YH, Hsia CY, Su CW, Lin HC, Loong CC, Chiou YY, Chiang JH, Lee PC, Huo TI, et al. A new prognostic model for hepatocellular carcinoma based on total tumor volume: the Taipei Integrated Scoring System. J Hepatol. 2010;53:108–117. doi: 10.1016/j.jhep.2010.01.038. [DOI] [PubMed] [Google Scholar]
- 37.Hsu CY, Huang YH, Su CW, Lin HC, Chiang JH, Lee PC, Lee FY, Huo TI, Lee SD. Renal failure in patients with hepatocellular carcinoma and ascites undergoing transarterial chemoembolization. Liver Int. 2010;30:77–84. doi: 10.1111/j.1478-3231.2009.02128.x. [DOI] [PubMed] [Google Scholar]
- 38.Min YW, Kim J, Kim S, Sung YK, Lee JH, Gwak GY, Paik YH, Choi MS, Koh KC, Paik SW, et al. Risk factors and a predictive model for acute hepatic failure after transcatheter arterial chemoembolization in patients with hepatocellular carcinoma. Liver Int. 2013;33:197–202. doi: 10.1111/liv.12023. [DOI] [PubMed] [Google Scholar]
- 39.Yu MC, Chan KM, Lee CF, Lee YS, Eldeen FZ, Chou HS, Lee WC, Chen MF. Alkaline phosphatase: does it have a role in predicting hepatocellular carcinoma recurrence? J Gastrointest Surg. 2011;15:1440–1449. doi: 10.1007/s11605-011-1537-3. [DOI] [PubMed] [Google Scholar]
- 40.Kim JM, Kwon CH, Joh JW, Park JB, Ko JS, Lee JH, Kim SJ, Park CK. The effect of alkaline phosphatase and intrahepatic metastases in large hepatocellular carcinoma. World J Surg Oncol. 2013;11:40. doi: 10.1186/1477-7819-11-40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Leung TW, Tang AM, Zee B, Lau WY, Lai PB, Leung KL, Lau JT, Yu SC, Johnson PJ. Construction of the Chinese University Prognostic Index for hepatocellular carcinoma and comparison with the TNM staging system, the Okuda staging system, and the Cancer of the Liver Italian Program staging system: a study based on 926 patients. Cancer. 2002;94:1760–1769. doi: 10.1002/cncr.10384. [DOI] [PubMed] [Google Scholar]
- 42.Chevret S, Trinchet JC, Mathieu D, Rached AA, Beaugrand M, Chastang C. A new prognostic classification for predicting survival in patients with hepatocellular carcinoma. Groupe d’Etude et de Traitement du Carcinome Hépatocellulaire. J Hepatol. 1999;31:133–141. doi: 10.1016/s0168-8278(99)80173-1. [DOI] [PubMed] [Google Scholar]
- 43.Zhuge Y, Zhang F, Qiu Y, Li Z, Zhang J. Prognostic accuracy of staging systems in patients with primary liver cancer undergoing transarterial chemoembolization. Hepatogastroenterology. 2013;60:481–488. doi: 10.5754/hge12003. [DOI] [PubMed] [Google Scholar]


