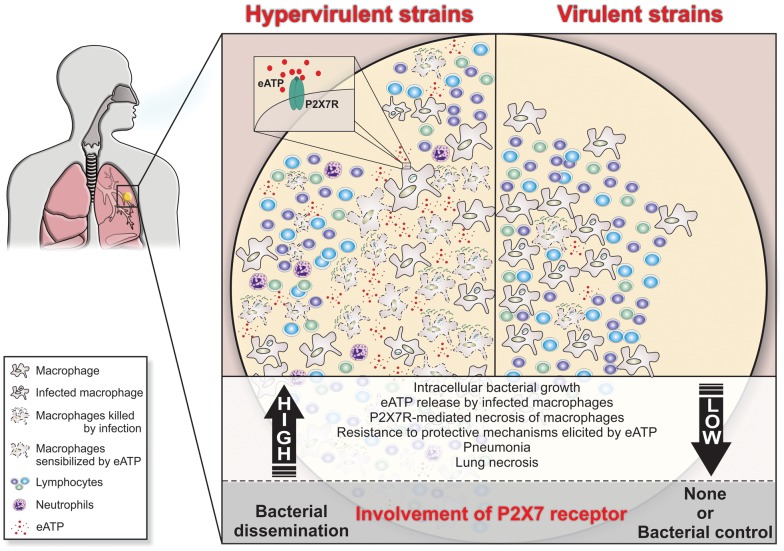
Figure 7. Schematic illustration of a hypothetical model to explain the high and low involvement of the P2X7R during severe and mild TB.
In severe TB, the rapid intracellular growth of hypervirulent mycobacteria results in massive macrophage damage. The eATP released by damaged cells may engage the P2X7R on their surfaces or on neighboring cells. The autocrine or paracrine P2X7R signaling cooperates with mycobacterial components exhibiting membrane-lysing activity and accelerates the necrotic death of infected macrophages and the spread of bacilli. The resistance of hypervirulent mycobacteria to the protective mechanisms elicited in macrophages by eATP contributes to disease dissemination. The release of large amounts of eATP triggers a vicious cycle that exacerbates the pulmonary recruitment of pathogen-permissive monocytes and macrophages and thereby leads to further intracellular bacillus growth. The suppressive environment that results from an excess of adenosine, a byproduct of eATP degradation, may also facilitate the survival of hypervirulent mycobacteria. According to this model, lung necrosis derives from programmed cell death that is triggered by P2X7R signaling. The modest levels of tissue damage induced by less virulent strains and the susceptibility of these mycobacteria to eATP-induced intracellular killing explain, respectively, the minor role and the protective effect of the P2X7R in the mild forms of TB.