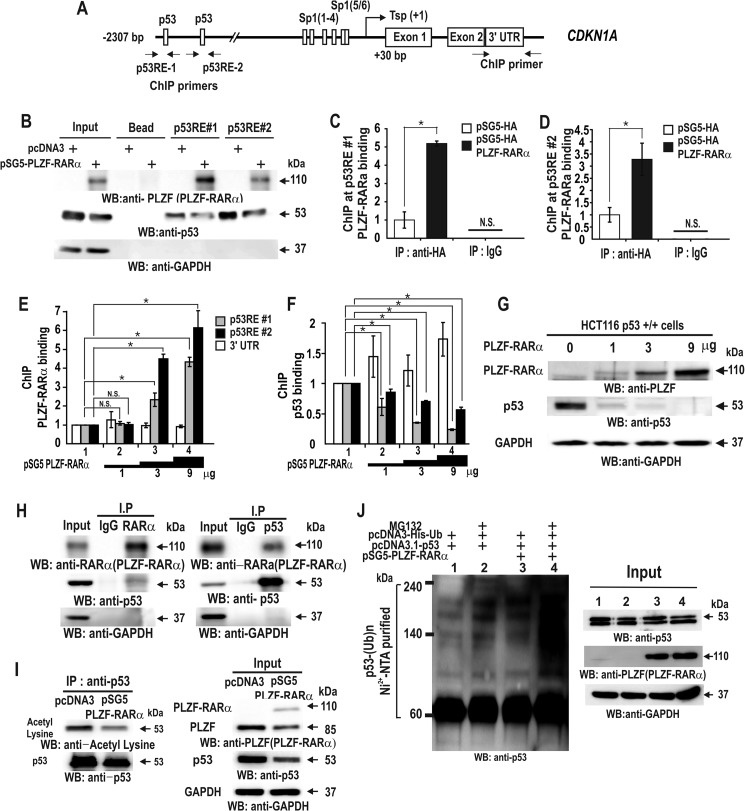
FIGURE 5.
PLZF-RARα represses CDKN1A gene transcription through binding competition with p53, TP53 transcriptional repression, and increased p53 ubiquitination. A, structure of the human CDKN1A gene promoter. The arrows at the p53 binding elements indicate the locations of the qChIP-PCR primer binding sites. Tsp(+1), transcription start site. B, oligonucleotide pulldown assay of PLZF-RARα binding to p53 response elements in the CDKN1A promoter. Extracts from HCT116 p53+/+ cells expressing ectopic PLZF-RARα were incubated with biotinylated oligonucleotides, incubated with streptavidin-agarose beads, and precipitated. The precipitates were analyzed by Western blot with the antibodies indicated. C–F, qChIP assay showing HA-PLZF-RARα/PLZF-RARα or p53 binding to the distal p53RE-1 and -2 of the endogenous CDKN1A gene in HCT116 p53+/+ cells. The cells were transfected with an increasing amount (0–9 μg) of pSG5-PLZF-RARα expression vector. Antibodies against PLZF, RARα, and p53 were used for ChIP. IgG, control. G, Western blot (WB) analyses showing PLZF-RARα and endogenous p53 expression in HCT116 p53+/+ cells transfected with an increasing amount (0–9 μg) of pSG5-PLZF-RARα expression vector. GAPDH, control. H and I, co-immunoprecipitation (IP) of PLZF-RARα and p53. HCT116 p53+/+ cell lysates were immunoprecipitated using an anti-RARα antibody and the precipitates were analyzed by Western blot using an anti-p53 antibody. Alternatively, the anti-p53 antibody was used first in the co-IP, and the anti-RARα antibody was used for Western blotting. J, ubiquitination assay for endogenous p53. H1299 cells were transfected with pcDNA3-His-ubiquitin or pSG5-PLZF-RARα in the various combinations indicated. The cells were cultured and treated with MG132 for 3 h prior to harvest. The cell lysates were then incubated with MagneHis nickel particles and the precipitated pellets were washed, resolved by 10% SDS-PAGE, and analyzed by Western blot using a p53 antibody. *, p < 0.05; N.S., not significant; t test.