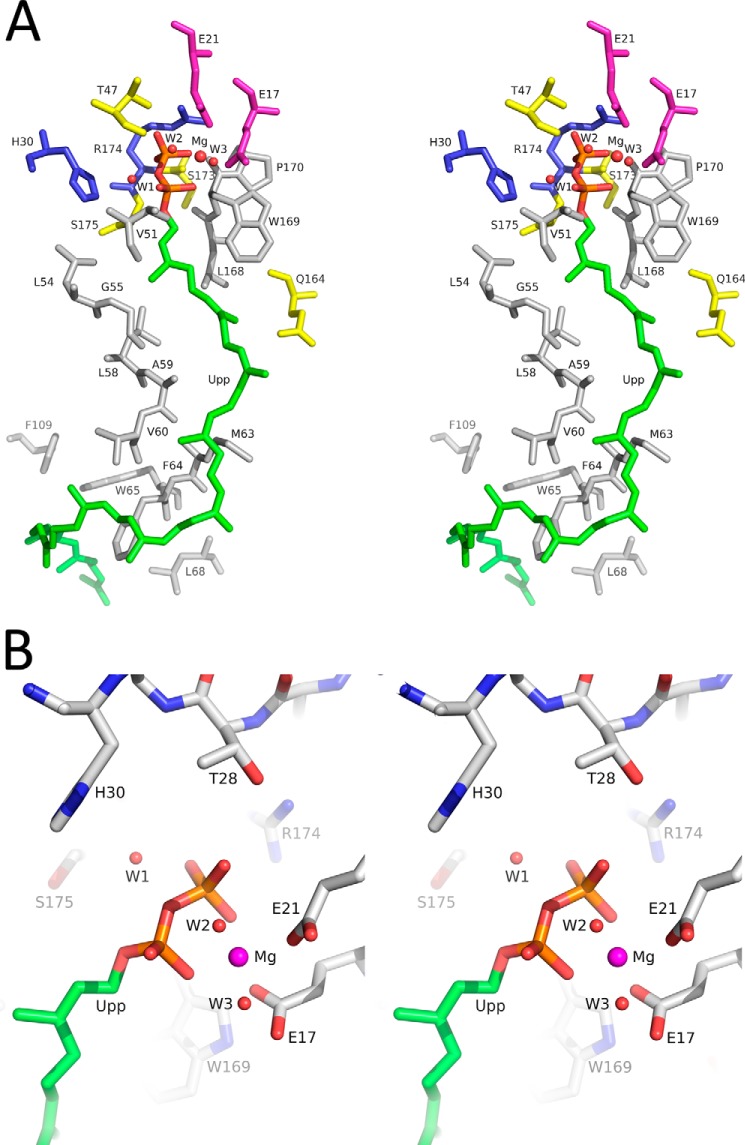
FIGURE 8.
Putative active site of E. coli UppP. A, residues surrounding the Upp substrate are represented. The nonpolar residues Val-51, Leu-54, Gly-55, Leu-58, Ala-59, Val-60, Met-63, Phe-64, Trp-65, Leu-68, Leu-168, Trp-169, and Pro-170 are in gray. The polar residues Thr-47, Gln-164, Ser-173, and Ser-175 are in yellow. The positively charged residues His-30 and Arg-174 are in blue. The negatively charged residues Glu-17 and Glu-21 are in purple. B, interactions between Upp pyrophosphate moiety and the nearby amino acids in the catalytic side are shown. The pyrophosphate moiety might be stabilized by Glu-17 and Glu-21 through a Mg2+ ion, and Arg-174 might interact with the α-phosphate of Upp directly. His-30 might catalyze through a water molecule in the reaction of hydrolysis.