FIGURE 2.
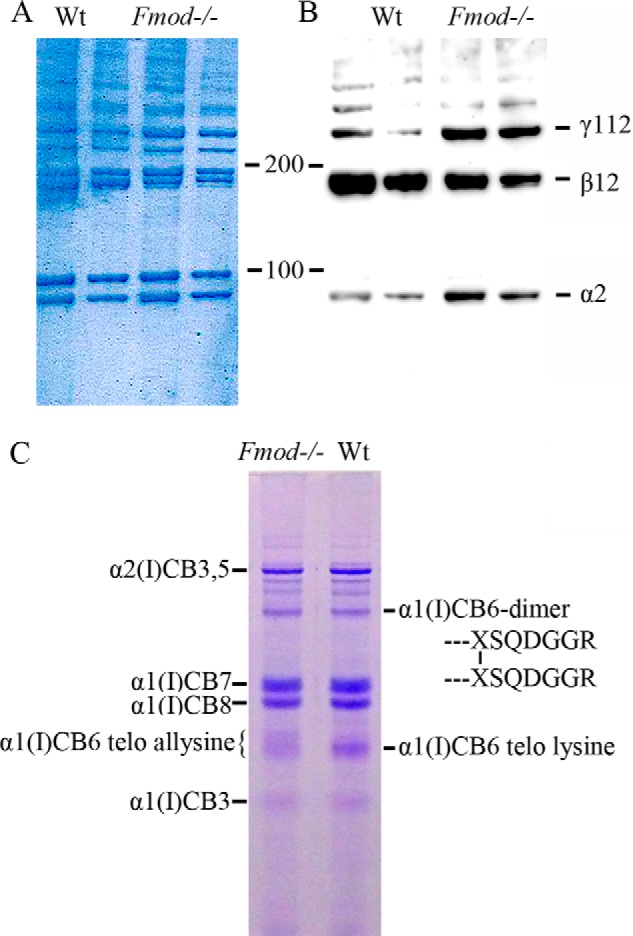
Enhanced stable cross-linking of α1(I) chains. A and B, acid-extracted collagen was run on 4–20% SDS-PAGE gradient gels and either stained with Coomassie Blue (A) or transferred to nitrocellulose membrane and immunoblotted with an antiserum specific to the collagen α2(I) chain (B). C, whole tendons were digested with CNBr, and the resulting collagen fragments were resolved on 12.5% SDS-PAGE staining with Coomassie Blue. The indicated α1(I)CB6-containing monomer and dimer bands were identified by in-gel trypsin digestion and mass spectroscopy. The prominent α1(I)CB6 monomer from wild-type tendon gave a C-terminal tryptic peptide cleaved after its C-terminal unmodified lysine residue. From Fmod−/− tendon, this component was largely missing, and the retarded, broad α1(I)CB6 monomer band gave a C-terminal tryptic peptide cleaved at arginine, six residues more C-terminal. Sequences of the latter same two tryptic peptides are shown later in Fig. 4.
