FIGURE 3.
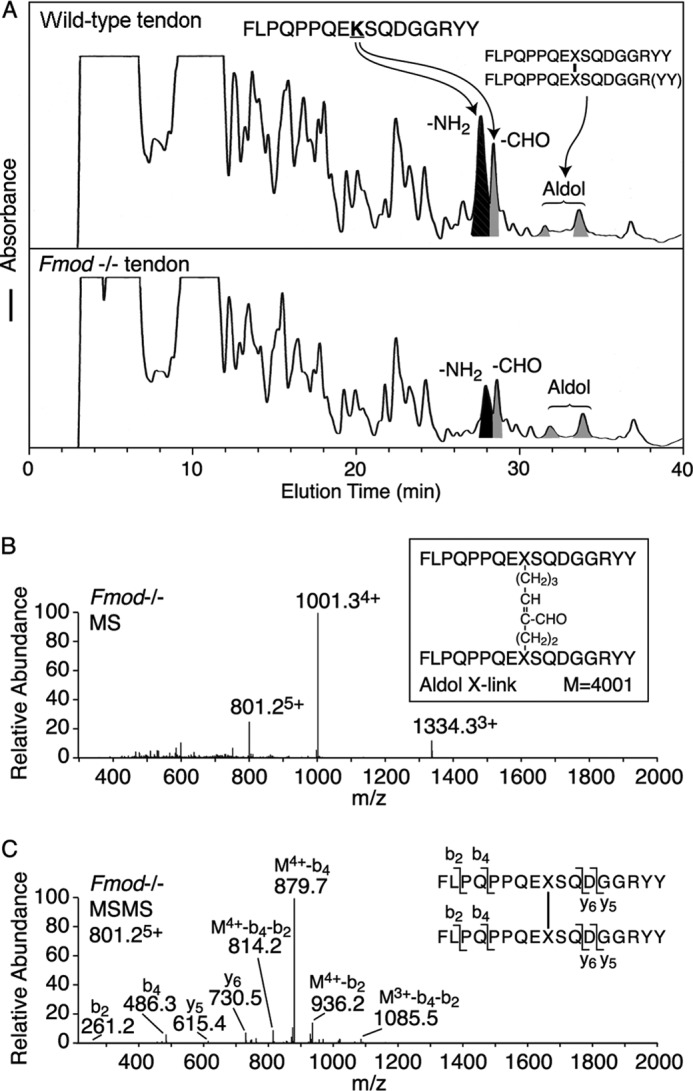
Mass spectrometric identification of the collagen type I C-telopeptide cross-linking domain. Bacterial collagenase-digested tendon collagen peptides from Fmod−/− and wild-type mice were resolved by HPLC (A) and identified by tandem mass spectrometry from their MSMS fragments (B and C). Consistently the ratio of unmodified lysine-containing C-telopeptide to aldehyde- plus aldol-containing C-telopeptides was approximately 2:1 from wild type compared with 1:2 from mutant tendon. The small peaks eluting from 25 to 27 min in wild-type tendon are amino and aldehyde versions of the C-telopeptide missing one or two Tyr residues from the C terminus. The structure of the aldol cross-link from Fmod−/− tendon was identified by MS (B) and MSMS fragmentation of the 801.25+ ion as shown (C). M4+-b4-b2 is a fragment of the parent ion with four charges that lost b2 from one arm and b4 from the other arm of the cross-linked structure.
