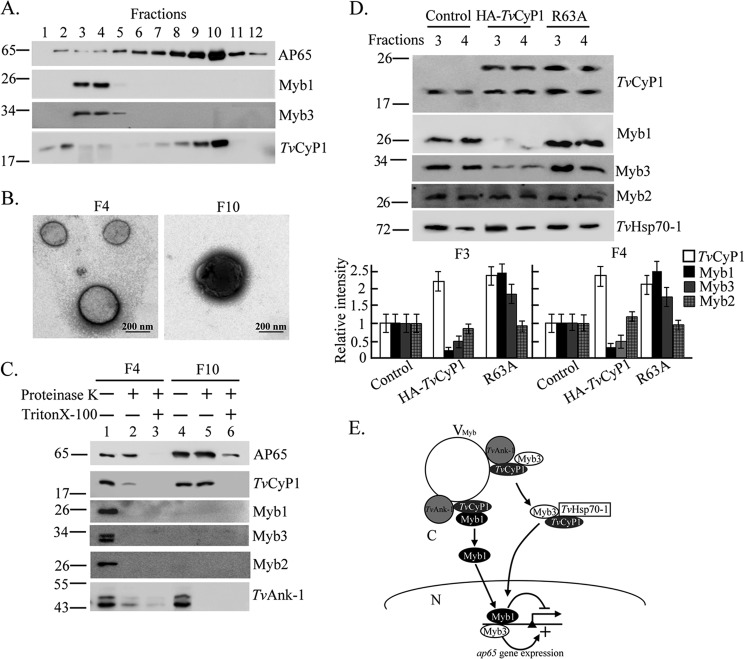
FIGURE 9.
Subcellular distribution of TvCyP1 and the trafficking of Myb1. In A, the P15 fraction from postnuclear lysates of T. vaginalis was separated by gradient centrifugation for Western blotting using the anti-Myb1, anti-Myb3, anti-TvCyP1, and 12G4 antibodies as indicated. In B, samples from fractions 4 and 10 were adsorbed onto Formvar-coated grids, fixed, negatively stained, and observed under a transmission electron microscope. The bar in the micrographs represents 200 nm. In C, samples from fractions 4 and 10 were examined by a protease protection assay in which samples were digested by the protease K with or without permeation by 0.5% Triton X-100 and examined by Western blotting using anti-TvCyP1, anti-Myb1, anti-Myb3, anti-Myb2, anti-TvAnk-1, and 12G4 antibodies as indicated. In D, vesicle fractions 3 and 4 from controls and cells overexpressing TvCyP1 and TvCyP1(R63A) were assayed by Western blotting using the antibodies to detect various proteins as indicated. The relative intensities of Myb1, Myb2, Myb3, and TvCyP1 versus TvHsp70-1 from three separate experiments are quantified in histograms. In E, the role of TvCyP1 in regulating the nuclear translocation of Myb1 and Myb3 is proposed. In this scheme, TvCyP1 may form a protein complex with TvAnk-1 and Myb1 or Myb3 on the outer surface of a novel vesicle, referred to as VMyb, to accelerate cis-trans interconversion of a glycinyl-prolyl bond in Myb1 and possibly in Myb3, resulting in the release of restrained Myb1 and Myb3 into the cytoplasm. In the cytoplasm, TvCyP1 forms a protein complex with TvHSP70-1 and Myb3 but not Myb1. Myb1 and Myb3 are then translocated into the nucleus where they regulate the transcription of the ap65-1 gene. Error bars represent S.D. from three separate experiments.