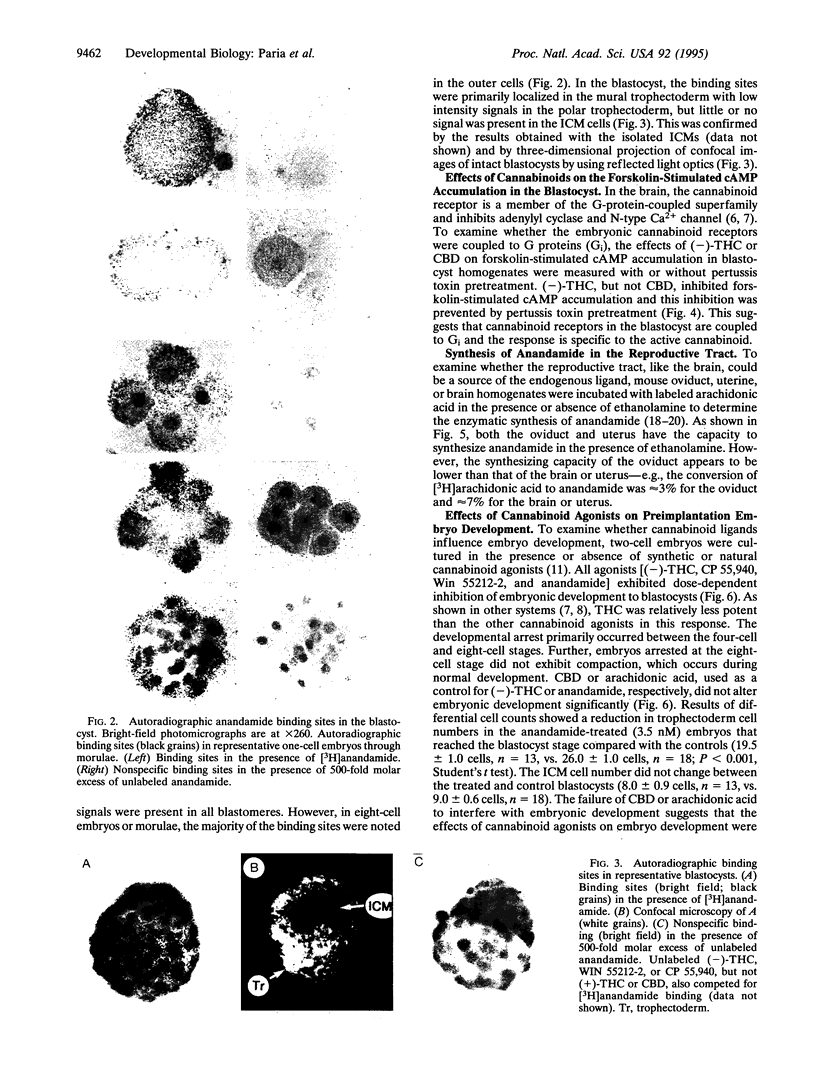
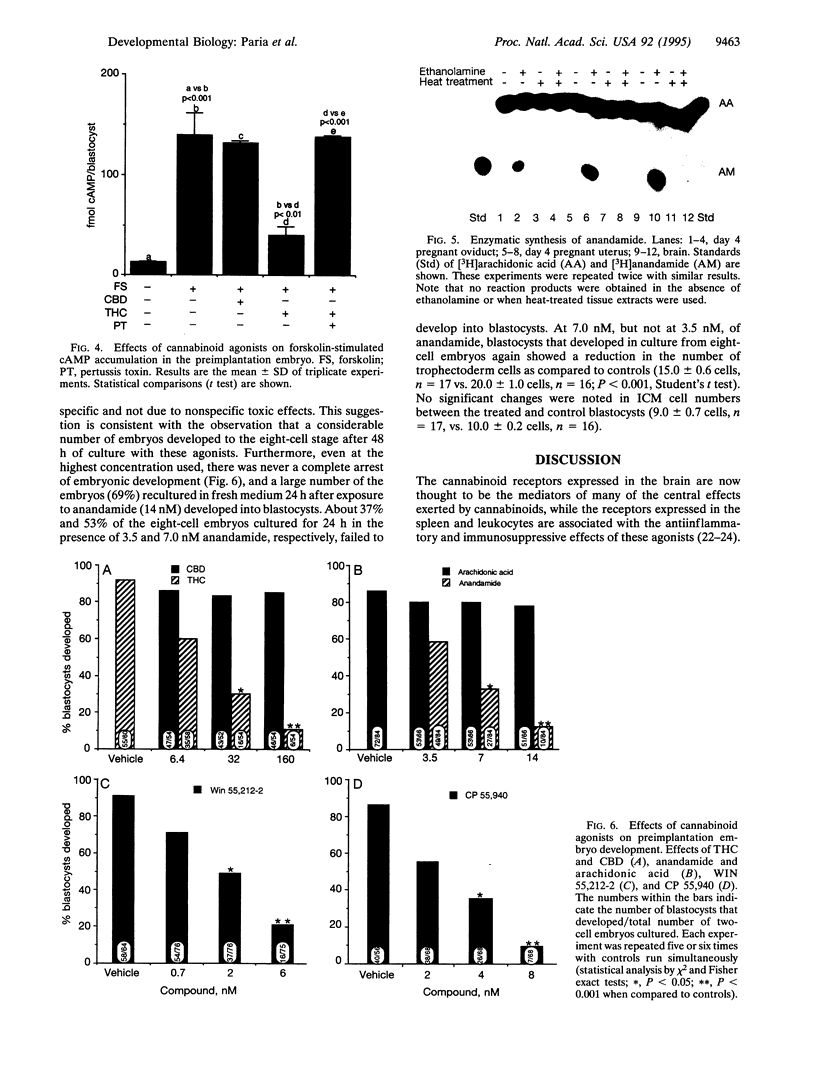
Abstract
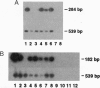
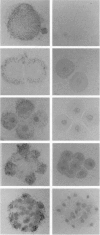
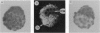
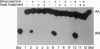
Using a reverse transcription-coupled PCR, we demonstrated that both brain and spleen type cannabinoid receptor (CB1-R and CB2-R, respectively) mRNAs are expressed in the preimplantation mouse embryo. The CB1-R mRNA expression was coincident with the activation of the embryonic genome late in the two-cell stage, whereas the CB2-R mRNA was present from the one-cell through the blastocyst stages. The major psychoactive component of marijuana (-)-delta-9-tetrahydrocannabinol [(-)-THC] inhibited forskolin-stimulated cAMP generation in the blastocyst, and this inhibition was prevented by pertussis toxin. However, the inactive cannabinoid cannabidiol (CBD) failed to influence this response. These results suggest that cannabinoid receptors in the embryo are coupled to inhibitory guanine nucleotide binding proteins. Further, the oviduct and uterus exhibited the enzymatic capacity to synthesize the putative endogenous cannabinoid ligand arachidonylethanolamide (anandamide). Synthetic and natural cannabinoid agonists [WIN 55,212-2, CP 55,940, (-)-THC, and anandamide], but not CBD or arachidonic acid, arrested the development of two-cell embryos primarily between the four-cell and eight-cell stages in vitro in a dose-dependent manner. Anandamide also interfered with the development of eight-cell embryos to blastocysts in culture. The autoradiographic studies readily detected binding of [3H]anandamide in embryos at all stages of development. Positive signals were present in one-cell embryos and all blastomeres of two-cell through four-cell embryos. However, most of the binding sites in eight-cell embryos and morulae were present in the outer cells. In the blastocyst, these signals were primarily localized in the mural trophectoderm with low levels of signals in the polar trophectoderm, while little or no signals were noted in inner cell mass cells.These results establish that the preimplantation mouse embryo is a target for cannabinoid ligands. Consequently, many of the adverse effects of cannabinoids observed during pregnancy could be mediated via these cannabinoid receptors. Although the physiological significance of the cannabinoid ligand-receptor signaling in normal preimplantation embryo development is not yet clear, the regulation of embryonic cAMP and/or Ca2+ levels via this signaling pathway may be important for normal embryonic development and/or implantation.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Asch R. H., Smith C. G. Effects of delta 9-THC, the principal psychoactive component of marijuana, during pregnancy in the rhesus monkey. J Reprod Med. 1986 Dec;31(12):1071–1081. [PubMed] [Google Scholar]
- Bouaboula M., Rinaldi M., Carayon P., Carillon C., Delpech B., Shire D., Le Fur G., Casellas P. Cannabinoid-receptor expression in human leukocytes. Eur J Biochem. 1993 May 15;214(1):173–180. doi: 10.1111/j.1432-1033.1993.tb17910.x. [DOI] [PubMed] [Google Scholar]
- Chang M. C., Berkery D., Schuel R., Laychock S. G., Zimmerman A. M., Zimmerman S., Schuel H. Evidence for a cannabinoid receptor in sea urchin sperm and its role in blockade of the acrosome reaction. Mol Reprod Dev. 1993 Dec;36(4):507–516. doi: 10.1002/mrd.1080360416. [DOI] [PubMed] [Google Scholar]
- Dalterio S., Bartke A. Fetal testosterone in mice: effect of gestational age and cannabinoid exposure. J Endocrinol. 1981 Dec;91(3):509–514. doi: 10.1677/joe.0.0910509. [DOI] [PubMed] [Google Scholar]
- Das S. K., Paria B. C., Andrews G. K., Dey S. K. Effects of 9-ene-tetrahydrocannabinol on expression of beta-type transforming growth factors, insulin-like growth factor-I and c-myc genes in the mouse uterus. J Steroid Biochem Mol Biol. 1993 Jun;45(6):459–465. doi: 10.1016/0960-0760(93)90160-x. [DOI] [PubMed] [Google Scholar]
- Das S. K., Paria B. C., Chakraborty I., Dey S. K. Cannabinoid ligand-receptor signaling in the mouse uterus. Proc Natl Acad Sci U S A. 1995 May 9;92(10):4332–4336. doi: 10.1073/pnas.92.10.4332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deutsch D. G., Chin S. A. Enzymatic synthesis and degradation of anandamide, a cannabinoid receptor agonist. Biochem Pharmacol. 1993 Sep 1;46(5):791–796. doi: 10.1016/0006-2952(93)90486-g. [DOI] [PubMed] [Google Scholar]
- Devane W. A., Axelrod J. Enzymatic synthesis of anandamide, an endogenous ligand for the cannabinoid receptor, by brain membranes. Proc Natl Acad Sci U S A. 1994 Jul 5;91(14):6698–6701. doi: 10.1073/pnas.91.14.6698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Devane W. A., Hanus L., Breuer A., Pertwee R. G., Stevenson L. A., Griffin G., Gibson D., Mandelbaum A., Etinger A., Mechoulam R. Isolation and structure of a brain constituent that binds to the cannabinoid receptor. Science. 1992 Dec 18;258(5090):1946–1949. doi: 10.1126/science.1470919. [DOI] [PubMed] [Google Scholar]
- Dewey W. L. Cannabinoid pharmacology. Pharmacol Rev. 1986 Jun;38(2):151–178. [PubMed] [Google Scholar]
- Di Marzo V., Fontana A., Cadas H., Schinelli S., Cimino G., Schwartz J. C., Piomelli D. Formation and inactivation of endogenous cannabinoid anandamide in central neurons. Nature. 1994 Dec 15;372(6507):686–691. doi: 10.1038/372686a0. [DOI] [PubMed] [Google Scholar]
- Dorris R. L., Lotzof L. Studies on lidocaine-induced kindling. Life Sci. 1992;51(1):25–28. doi: 10.1016/0024-3205(92)90214-a. [DOI] [PubMed] [Google Scholar]
- Ducibella T., Anderson E. Cell shape and membrane changes in the eight-cell mouse embryo: prerequisites for morphogenesis of the blastocyst. Dev Biol. 1975 Nov;47(1):45–58. doi: 10.1016/0012-1606(75)90262-6. [DOI] [PubMed] [Google Scholar]
- Felder C. C., Briley E. M., Axelrod J., Simpson J. T., Mackie K., Devane W. A. Anandamide, an endogenous cannabimimetic eicosanoid, binds to the cloned human cannabinoid receptor and stimulates receptor-mediated signal transduction. Proc Natl Acad Sci U S A. 1993 Aug 15;90(16):7656–7660. doi: 10.1073/pnas.90.16.7656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilman A. G., Murad F. Assay of cyclic nucleotides by receptor protein binding displacement. Methods Enzymol. 1974;38:49–61. doi: 10.1016/0076-6879(74)38010-x. [DOI] [PubMed] [Google Scholar]
- Gérard C. M., Mollereau C., Vassart G., Parmentier M. Molecular cloning of a human cannabinoid receptor which is also expressed in testis. Biochem J. 1991 Oct 1;279(Pt 1):129–134. doi: 10.1042/bj2790129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hardy K., Handyside A. H., Winston R. M. The human blastocyst: cell number, death and allocation during late preimplantation development in vitro. Development. 1989 Nov;107(3):597–604. doi: 10.1242/dev.107.3.597. [DOI] [PubMed] [Google Scholar]
- Jones J., Schultz R. M. Pertussis toxin-catalyzed ADP-ribosylation of a G protein in mouse oocytes, eggs, and preimplantation embryos: developmental changes and possible functional roles. Dev Biol. 1990 Jun;139(2):250–262. doi: 10.1016/0012-1606(90)90294-s. [DOI] [PubMed] [Google Scholar]
- Kaminski N. E., Abood M. E., Kessler F. K., Martin B. R., Schatz A. R. Identification of a functionally relevant cannabinoid receptor on mouse spleen cells that is involved in cannabinoid-mediated immune modulation. Mol Pharmacol. 1992 Nov;42(5):736–742. [PMC free article] [PubMed] [Google Scholar]
- Kruszka K. K., Gross R. W. The ATP- and CoA-independent synthesis of arachidonoylethanolamide. A novel mechanism underlying the synthesis of the endogenous ligand of the cannabinoid receptor. J Biol Chem. 1994 May 20;269(20):14345–14348. [PubMed] [Google Scholar]
- Manejwala F., Kaji E., Schultz R. M. Development of activatable adenylate cyclase in the preimplantation mouse embryo and a role for cyclic AMP in blastocoel formation. Cell. 1986 Jul 4;46(1):95–103. doi: 10.1016/0092-8674(86)90863-9. [DOI] [PubMed] [Google Scholar]
- Matsuda L. A., Lolait S. J., Brownstein M. J., Young A. C., Bonner T. I. Structure of a cannabinoid receptor and functional expression of the cloned cDNA. Nature. 1990 Aug 9;346(6284):561–564. doi: 10.1038/346561a0. [DOI] [PubMed] [Google Scholar]
- Munirathinam G., Yoburn B. C. A simple procedure for assaying cAMP. Pharmacol Biochem Behav. 1994 Jul;48(3):813–816. doi: 10.1016/0091-3057(94)90352-2. [DOI] [PubMed] [Google Scholar]
- Munro S., Thomas K. L., Abu-Shaar M. Molecular characterization of a peripheral receptor for cannabinoids. Nature. 1993 Sep 2;365(6441):61–65. doi: 10.1038/365061a0. [DOI] [PubMed] [Google Scholar]
- Nahas G., Latour C. The human toxicity of marijuana. Med J Aust. 1992 Apr 6;156(7):495–497. [PubMed] [Google Scholar]
- Pacheco M., Childers S. R., Arnold R., Casiano F., Ward S. J. Aminoalkylindoles: actions on specific G-protein-linked receptors. J Pharmacol Exp Ther. 1991 Apr;257(1):170–183. [PubMed] [Google Scholar]
- Pakrasi P. L., Dey S. K. Role of calmodulin in blastocyst formation in the mouse. J Reprod Fertil. 1984 Jul;71(2):513–517. doi: 10.1530/jrf.0.0710513. [DOI] [PubMed] [Google Scholar]
- Paria B. C., Das S. K., Andrews G. K., Dey S. K. Expression of the epidermal growth factor receptor gene is regulated in mouse blastocysts during delayed implantation. Proc Natl Acad Sci U S A. 1993 Jan 1;90(1):55–59. doi: 10.1073/pnas.90.1.55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paria B. C., Dey S. K. Preimplantation embryo development in vitro: cooperative interactions among embryos and role of growth factors. Proc Natl Acad Sci U S A. 1990 Jun;87(12):4756–4760. doi: 10.1073/pnas.87.12.4756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poueymirou W. T., Schultz R. M. Regulation of mouse preimplantation development: inhibition of synthesis of proteins in the two-cell embryo that require transcription by inhibitors of cAMP-dependent protein kinase. Dev Biol. 1989 Jun;133(2):588–599. doi: 10.1016/0012-1606(89)90061-4. [DOI] [PubMed] [Google Scholar]
- Schuel H., Goldstein E., Mechoulam R., Zimmerman A. M., Zimmerman S. Anandamide (arachidonylethanolamide), a brain cannabinoid receptor agonist, reduces sperm fertilizing capacity in sea urchins by inhibiting the acrosome reaction. Proc Natl Acad Sci U S A. 1994 Aug 2;91(16):7678–7682. doi: 10.1073/pnas.91.16.7678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Temeles G. L., Ram P. T., Rothstein J. L., Schultz R. M. Expression patterns of novel genes during mouse preimplantation embryogenesis. Mol Reprod Dev. 1994 Feb;37(2):121–129. doi: 10.1002/mrd.1080370202. [DOI] [PubMed] [Google Scholar]