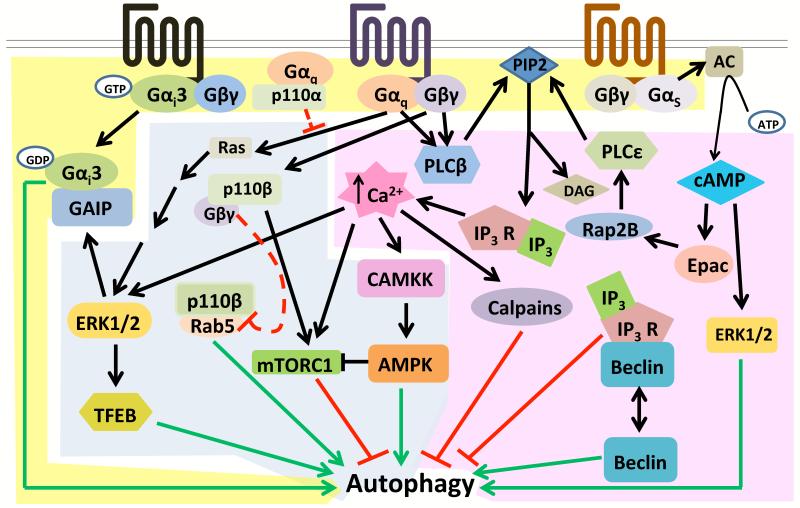
Figure 1. Multiple signals downstream of GPCRs regulate autophagy.
(A) (Highlighted in yellow) Dissociated subunits of heterotrimeric G proteins regulate autophagy: Liberated GDP-bound Gαi3 has been shown to induce autophagy in a VPS34-dependent manner, which is promoted by the RGS family protein GAIP. Gβγ subunits liberated by Gq-coupled receptors activate PLCβ, which cleaves PIP2 to generate IP3 and DAG and IP3 induces calcium release from the ER. (B) (Highlighted in pink) Second messengers generated by GPCR activation modulate autophagy: Gs-coupled receptor stimulation activates adenylyl cyclase (AC) which generates cAMP from ATP. cAMP increases autophagy in some cell types but reduces it in others. While enhanced autophagy can be ERK1/2 dependent, inhibition of autophagy can involve IP3 generated by PLCε. IP3 can negatively regulate autophagy through calcium-activated CAMKKβ signaling and/or via association of IP3R with Beclin-1. Like cAMP, increased cytosolic calcium can decrease autophagy in some contexts but increase it in others. Calcium can induce autophagy via CAMKK and/or ERK1/2 signaling, and reduce autophagy through calpains. (C) (Highlighted in blue) Kinases downstream of GPCRs regulate autophagy: The MAPKs ERK1/2 are stimulated by GPCRs. ERK1/2 can promote autophagy during starvation by phosphorylating the transcription factor TFEB, causing its translocation to the nucleus and accelerated autophagic and lysosomal gene expression. ERK2 also activates GAIP to increase Gαi3-induced autophagy. CAMKKβ activated by Gq/11 and Gs-coupled receptor stimulation can activate the critical autophagy regulator AMPK. The class 1A PI3 kinase p110β binds the endosomal trafficking regulator Rab5 to promote autophagy. Gα/p110α and Gβγ/p110β complexes likely inhibit autophagy.