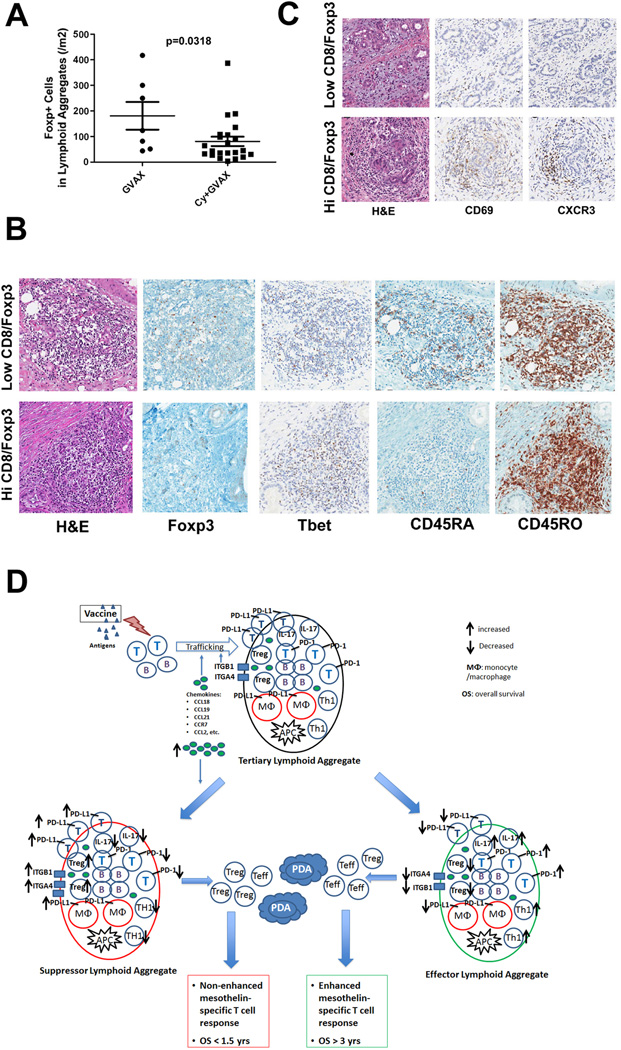
Figure 7. Low-dose cyclophosphamide reduces intratumoral Treg numbers and promotes enhanced T-cell trafficking and activation within the tertiary lymphoid aggregates.
A. Comparison of the densities of Foxp3+ T cells in lymphoid aggregates between patients receiving GVAX alone (GVAX) and patients receiving GVAX plus Treg-modulating doses of Cy (CAX+Cy). B. IHC staining of Foxp3, T-bet, CD45RA, and CD45RO in representative lymphoid aggregates in PDACs from patients with high Teffector:Treg ratios in the TME compared to those with low Teffector:Treg ratios in the TME. C. IHC staining of CD69 and CXCR3 in representative PDAC tumor areas from patients with high versus low Teffector:Treg ratios in the TME. All positive IHC signals are in brown. D. Proposed model of vaccine-induced T-cell infiltration into the PDAC TME. At baseline, the “non-immunogenic” PDAC TME is primarily immunosuppressed and infiltrated with Tregs and other immunosuppressive populations of innate immune cells, and low numbers of effector T cells. Vaccination induces antigen-specific T cells systemically that traffic to the TME and initiate an inflammatory reaction. Chemokines including CCL2, CCL18, CCL19 and CCL21, and other signals produced during this inflammatory reaction recruit additional immune effector and regulatory cells, and induce the neogenesis of tertiary lymphoid aggregates within the TME, converting the PDAC TME into an “immunogenic” environment. These lymphoid aggregates are composed of effector T cells, B cells, Tregs and antigen-presenting cells (APCs), and serve as nodal sites for immune activation and regulation within the TME. Paradoxically, higher expression of the chemokines associated with the development of these aggregates favors the recruitment of immunosuppressive immune cells into the lymphoid aggregates in the TME. Lymphoid aggregates dominated by effector T cells and immune activation signals (“Effector Lymphoid Aggregates”) including: increased CD8+ effector T cells and effector T cells with Th17 signatures, increased PD-1 expression on effector T cells, decreased Tregs, and lower expression of PD-L1, are associated with higher Teffector:Treg ratios in TIL and enhanced post-vaccination T-cell responses in PBL, and generate productive antitumor responses that can prolong survival. In contrast, lymphoid aggregates dominated by immunosuppressive immune cells and signals (“Suppressor Lymphoid Aggregates”) including: decreased CD8+ effector T cells and effector T cells with Th17 signatures, decreased PD-1 expression on effector T cells, increased Tregs, and higher expression of PD-L1, are associated with decreased T effector:Treg ratios in TIL and unenhanced T-cell responses in PBL, do not generate productive antitumor responses, and cannot prolong survival. Use of targeted immune modulators may tip the balance between these signals in favor of the development of “effector lymphoid aggregates” and enhance the intratumoral immune responses induced by vaccines. However, without a vaccine, an “immunogenic” TME containing effector T cells and lymphoid aggregates is not available for immune modulators to act on.