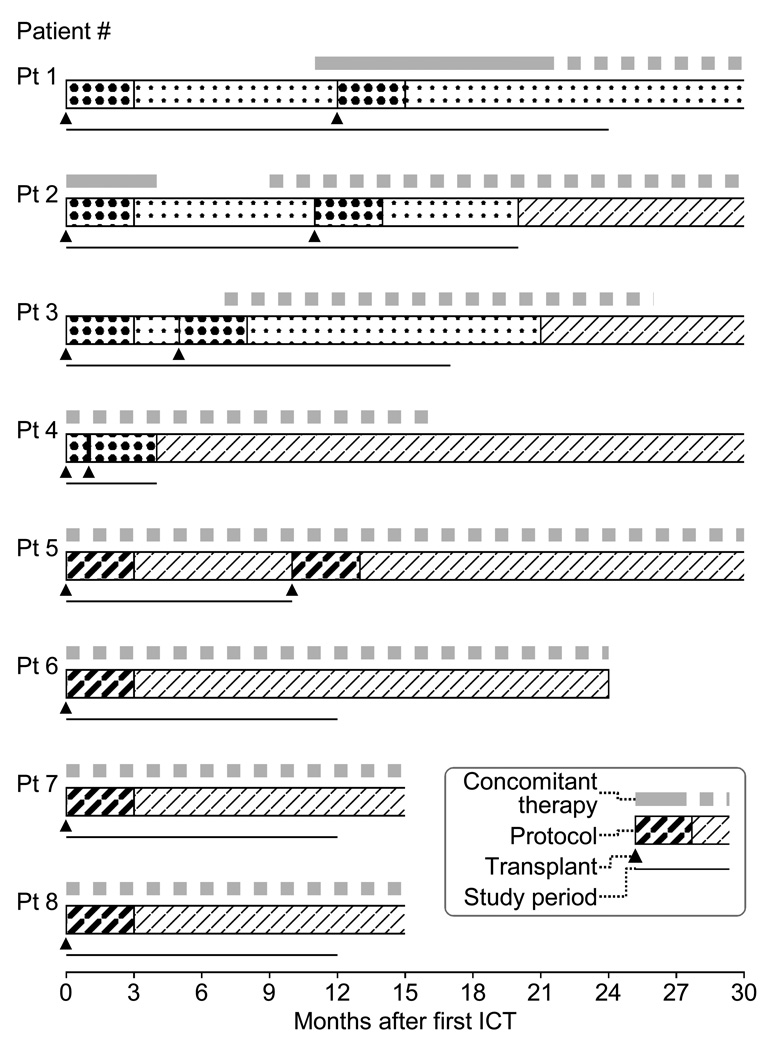
Figure 3.
Immunosuppression for each patient.
Clinical course of immunosuppression for each patient is shown as follows from upper row: concomitant therapy (exenatide; gray solid line and sitagliptin; gray dotted line), immunosuppression protocol (induction therapy using Edmonton-type protocol; densely dotted area, maintenance therapy using Edmonton-type protocol; lightly dotted area, induction therapy using TCD-AI protocol; densely slashed area and maintenance therapy using TCD-AI protocol; lightly slashed area), islet cell transplantation (triangle) and the period included in this study (black narrow line). Immunosuppression maintenance for patient 1 was changed to the TCD-AI protocol 34 months after the initial ICT.