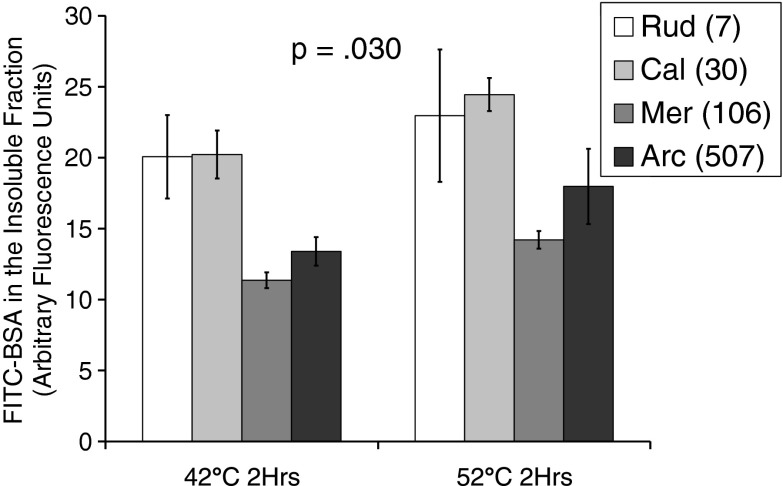
Fig. 4.
FITC-tagged BSA aggregation: using the same reporter protein in the lysates of our various species allowed us to investigate the potential role of molecular chaperones and co-chaperones in the protein stability properties of our species. Data represents BSA aggregates in the pellet after 2-h temperature stress at 42 or 52 °C and 1-h ultracentrifugation at 100,000 g. The two shorter-lived species clustered together and aggregated more BSA than the two longer-lived species, which also clustered together. Results were assessed by two-way analysis of variance, indicating a significant main effect of species, F(3,15) = 3.90, p = .030, but no difference between 42 and 52 °C, F(1,15) = .788, p = .389. There was also no interaction effect, F(3,15) = .068, p = .976