Abstract
Background
Reduced brain-derived neurotrophic factor (BDNF) levels have been reported in the serum and plasma of patients with psychosis. The aim of this study was to investigate potential causes and consequences of reduced BDNF expression in these patients, by examining the association between BDNF levels and measures of stress, inflammation and hippocampal volume in first-episode psychosis.
Methods
BDNF, interleukin (IL)-6, and tumour-necrosis-factor (TNF) alpha mRNA levels were measured in leukocytes of 49 first-episode psychosis patients (DSM-IV criteria) and 30 healthy controls, recruited between January 2006 and December 2008. In the same subjects, we measured salivary cortisol levels, and collected information about psychosocial stressors (number of childhood trauma, number of recent stressors, and perceived stress). Finally, hippocampal volume was measured, using brain MRI, in a subsample of 19 patients.
Results
Patients had reduced BDNF (effect size d=1.3, p<0.001) and increased IL-6 (effect size d=1.1, p<0.001) and TNF-alpha (effect size d=1.7, p<0.001) gene expression levels, when compared with controls, as well as higher levels of psychosocial stressors. A linear regression analysis in patients showed that a history of childhood trauma and high levels of recent stressors predicted lower BDNF expression through an inflammation-mediated pathway (adjusted R square=0.23, p=0.009). In turn, lower BDNF expression, increased IL-6 expression, and increased cortisol levels, all significantly and independently predicted a smaller left hippocampal volume (adjusted R square=0.71, p<0.001).
Conclusions
Biological changes activated by stress represent a significant factor influencing brain structure and function in first-episode psychosis, through an effect on BDNF.
Introduction
Several studies have reported reduced brain-derived neurotrophic factor (BDNF) levels in the brain 1 and in the serum and plasma of patients with schizophrenia 2-7. BDNF is widely expressed in the adult mammalian brain and is known to play a crucial role promoting proliferation, regeneration and survival of neurons 8. This is particularly important in this context, since a number of studies has shown brain volume changes at the onset of psychosis, or during the transition to psychosis, suggesting a critical role for neuroplasticity, especially in the hippocampus, in the development of psychosis 9-11. Moreover, BDNF has also been involved in more complex processes in adulthood, such as regulation of cognitive function 12 that is known to be impaired in patients with psychosis 13;14. However, the clinical and biological mechanisms behind the lower BDNF levels and the progressive brain volume changes in psychosis are still unknown.
Stress and the biological systems involved in the stress response have been suggested to play a role in BDNF changes. Indeed, animal models have shown that chronic stress down-regulates hippocampal BDNF mRNA expression and impairs processes of neuronal branching and neurogenesis 15. High levels of both glucocorticoid hormones and pro-inflammatory cytokines, two key players in the response to stress, have been associated with decreased BDNF levels 16;17 and with changes in hippocampal neuronal function such as dendrite atrophy, neuronal death and reduced neurogenesis 18-21. Interestingly, we and others have shown both abnormal glucocorticoid function and increased inflammatory markers in patients with first-episode psychosis 22;23. Furthermore, we have recently shown a negative correlation between cortisol levels and left hippocampal volume in first-episode psychosis 24. Previous studies have also shown that childhood trauma, a powerful psychosocial stressor, is associated with smaller left hippocampal volume in patients with other psychiatric disorders such as depression and post-traumatic stress disorder 25;26. However, the potential pathways linking stressful events to BDNF and to hippocampal volume have never been studied in psychosis.
In the present study, we aim to investigate the pathways leading from psychosocial stress to reduced BDNF levels in patients with first-episode psychosis, and to evaluate the putative consequence of these reduced BDNF levels on hippocampal volumes. Of relevance, BDNF in the blood seems to reflect the BDNF content in the brain: this notion is substantiated by animal experiments, showing that BDNF serum levels are correlated with BDNF expression in cortical and hippocampal brain regions 27-29, and further supported by a positive correlation between plasma and cerebrospinal fluid (CSF) BDNF levels recently reported in drug naïve first-episode psychosis patients 30. We have specifically measured BDNF expression in leukocytes, as leukocyte gene expression profile shows similarities to those observed in the brain, especially for genes linked to neurotransmitters, cytokines, hormones, and growth factors 29;31. BDNF mRNA expression has been recently found to be decreased in leukocytes of depressed patients 32: however, this has never been studied in psychosis. Moreover, we have measured 1) psychosocial stress (childhood trauma, recent stressful events and perceived stress); 2) inflammatory markers (leukocyte mRNA levels of interleukin (IL-6) and tumour-necrosis factor (TNF)-alpha); 3) diurnal salivary cortisol levels; and 4) hippocampal volume (via Magnetic Resonance Imaging, MRI). Some of the subjects described in this paper belongs to a group of patients and controls previously described 22;24. This paper is now advancing this work, presenting for the first time the data on BDNF gene expression and on inflammatory markers in these patients, and the association between these variables and the stress and imaging measures.
Methods
Subjects
First-episode psychosis patients were recruited in London (UK) from inpatient and outpatient units, part of the South London and Maudsley (SLAM) NHS Foundation Trust in South-East London (UK), from January 2006 to December 2008. The recruitment strategy was based on contacting inpatients and outpatients services interviewing staff and reviewing clinical notes, and approaching all subjects aged 18-65 who presented for the first time to these services for a functional psychotic illness (ICD10 F10-19, excluding coding F1x.0 for Acute intoxication; F20-29 and F30-39, psychotic codings) 33. Patients with organic psychosis, learning disabilities or not fluent in English were excluded from the study. Controls were recruited from the same catchment area as the patients through advertisement in local newspapers, hospitals and job centers, as well as from existing volunteer databases. Controls were screened using the Psychosis Screening Questionnaire (PSQ) 34, and excluded if they met criteria for a present or past psychotic disorder. The study was approved by the local Research Ethics Committee, in accordance with the code of ethics of the World Medical Association, and written informed consent was obtained from all participants.
We recruited and assessed 49 patients with first-episode psychosis and 30 healthy controls. All subjects had an assessment of BDNF, IL-6 and TNF-alpha expression; only 19 of these patients accepted to undergo an MRI scan. Twenty-seven patients received a DSM-IV diagnosis of schizophrenia/schizophreniform disorder, 15 schizoaffective or affective psychosis, and 7 psychotic disorder not otherwise specified. Ten patients were drug naïve, 36 were on an atypical antipsychotic (mostly olanzapine, n-19), and 3 on a typical antipsychotic. The mean duration of antipsychotic treatment was 33.4±5.8 days (range from 0 to 170 days).
Questionnaires and Clinical Assessment
Validation of clinical diagnosis was obtained using the Operational Criteria (OPCRIT) 35, reviewing the case notes in the first month following first contact with services. We collected information about stressful life events occurred in the previous six months using the Brief Life Events questionnaire 36, and measured perceived stress in the previous month using the Perceived Stress Scale 37. Information about childhood trauma were also collected, using a modified version of the Childhood Experience of Care and Abuse (CECA) Questionnaire 38. Please see supplemental material for details on childhood trauma assessment.
Gene expression analyses
Blood samples for gene expression of BDNF, IL-6 and TNF-alpha were collected using PaxGene Tubes. The time of blood collection varied for each subject, and subjects did not fast before collection. After blood samples were withdrawn, PaxGene Tubes were kept for two hours at room temperature and then stored at −80°C until they were processed. RNA isolation was performed using the PAXGene Blood RNA Kit (Qiagen S.p.A., Milan, MI, Italy) according to manufacturer’s protocols. Please see supplemental material for details on gene expression analyses.
Salivary cortisol assessment
Saliva samples were collected to measure salivary cortisol using Salivettes (Sarstedt, Leicester, UK). Methods of sample collection and analyses have been described in detail before 22. Subjects were instructed to collect saliva samples immediately after awakening and again at 12 pm and at 8pm. Cortisol levels during the day were measured as Area Under the Curve (AUC) of cortisol levels at the three time points, following the calculation of the AUC derived from the trapezoid formula 39.
Hippocampal volume
Magnetic resonance imaging scans were acquired on 19 of the recruited patients with a GE Signa 1.5-T system (GE Medical Systems, Milwaukee), at the Maudsley Hospital, London. Please see supplemental material for details on MRI scans acquisition. Hippocampal volume was measured blind to group status or BDNF, cytokine and cortisol levels, by one single rater using the software program MEASURE (version 0.8, Johns Hopkins University, Baltimore, MD). This image analysis program uses stereologically unbiased estimation of volume. The program and the measurement procedure have been described in detail elsewhere 40.
Data Analyses
Data were analyzed using the Statistical Package for Social Sciences, Version 15.0 (SPSS Inc.). Continuous variables are presented as mean ± standard error mean. Chi-square test was used to compare categorical variables between patients and controls. Independent T-test was applied to test differences in BDNF expression and other continuous variables between patients and controls. Parametric and non-parametric correlation analyses were used, as appropriate, to test the association between BDNF expression and psychosocial stress, inflammatory markers and cortisol levels, separately in patients and controls. A linear regression model was then used to test for predictors of BDNF expression and of hippocampal volume in patients.
Results
Socio-demographic variables, psychosocial stress measures and biological variables, in first-episode psychosis patients and healthy controls, are shown in Table 1. Patients with first-episode psychosis showed reduced BDNF gene expression (effect size d=1.3) and increased IL-6 (d=1.1) and TNF-alpha (d=1.7) gene expression, compared with controls. Consistent with the data previously described in a sample comprising some of the same subjects 22, measures of psychosocial stress were significantly higher in patients than in controls (Table 1), and cortisol levels were similar in patients and controls (Table 1).
Table 1.
Socio-demographic characteristics, stress variables, IL-6, TNF-alpha and BDNF gene expression of first-episode psychosis patients and healthy controls.
| Patients n=49 | Controls n=30 | Test and significance | |
|---|---|---|---|
| Age (years) | 28.2±0.9 | 27.0±0.8 | t=−1.0, df=1, 77, p=0.3 |
| Gender (M/F) | (33/16) | (19/11) | χ2=0.1, p=0.8 |
| % of males | 67.3% | 63.3% | |
| Body Mass Index (kg/m2) | 24.8±0.8 | 23.5±0.8 | t=−1.2, df=1, 60, p=0.3 |
| Ethnicity (White/Others) | (11/38) | (9/21) | χ2=0.6, p=0.6 |
| % of white British | 22.4% | 30% | |
| N. of recent stressors | 2.3±0.2 | 1.3±0.2 | t=−3.0, df=1, 71, p=0.004 |
| Perceived Stress Scale | 21.4±1.1 | 12.0±1.2 | t=−5.6, df=1, 71, p<0.001 |
| Childhood trauma (no/yes) | (9/32) | (17/11) | χ2=10.7, p=0.002 |
| % with at least 1 trauma | 78% | 39.3% | |
| Day cortisol (nmol hour/l) | 79.2±4.6 | 77.6±7.7 | t=−0.2, df=1, 43, p=0.9 |
| IL-6 expression | 1.67±0.10 | 1.04±0.09 | t=−4.6, df=1, 73, p<0.001 |
| TNF-alpha expression | 1.66±0.06 | 1.04±0.05 | t=−7.7, df=1, 75, p<0.001 |
| BDNF expression | 0.54±0.05 | 1.17±0.10 | t=5.5, df=1, 77, p<0.001 |
Correlation analyses with BDNF gene expression
We ran exploratory correlation analyses between BDNF gene expression levels and both the immune markers and the psychosocial stress measures, in both patients and controls. In patients, childhood trauma and number of recent stressful life events were negatively correlated with BDNF mRNA levels (respectively Spearman’s rho=-0.42, p=0.006, and Spearman’s rho=-0.32, p=0.03). IL-6 gene expression was also significantly negatively correlated with BDNF gene expression (Pearson’s r=-0.33, p=0.02). Because of the presence of one outlier for IL-6 mRNA levels, we also re-run the analysis without this subject and the correlation remained significant (r=-0.29, p=0.05). There was no significant correlation between BDNF mRNA levels and duration of antipsychotic treatment (see Supplemental Material, Table S1).
In the control group, there was a trend for a negative correlation between number of recent stressful events and BDNF gene expression (Spearman’s rho=-0.35, p=0.06). None of the other variables was correlated with BDNF gene expression in the control group (see Supplemental Material, Table S1).
Multiple Linear Regression Analyses for BDNF gene expression in first-episode psychosis
In order to establish possible predictors of BDNF gene expression in patients with first-episode psychosis, we ran a linear regression analysis including all the variables identified statistically to be correlated with BDNF mRNA levels. The first model included only the psychosocial stress variables (the number of childhood trauma and the number of recent stressful events) and accounted for 21% of the variance in BDNF gene expression (adjusted R square, p=0.006). Adding IL-6 gene expression to the model did not increase the variance explained, suggesting that the effects of psychosocial stressors on BDNF may be mediated by the increased IL-6; in fact, a second model including number of childhood trauma, number of recent stressful events and IL-6 gene expression accounted for 23% of the variance in BDNF gene expression (adjusted R square, p=0.009). See Table 2.
Table 2.
Multiple Linear Regression for BDNF expression in first-episode psychosis patients.
| R | Adjusted R square | Significance | ||
|---|---|---|---|---|
| 1st model | Childhood Trauma + Recent stressful events | 0.51 | 0.21 | p=0.006 |
| 2nd model | Childhood Trauma + Recent stressful events + IL-6 expression | 0.54 | 0.23 | p=0.009 |
Correlation analyses with left hippocampal volume in first-episode psychosis
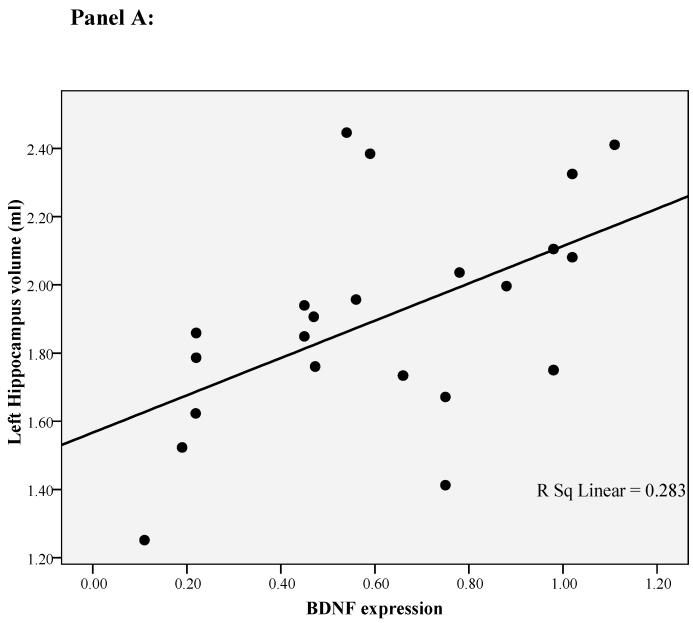
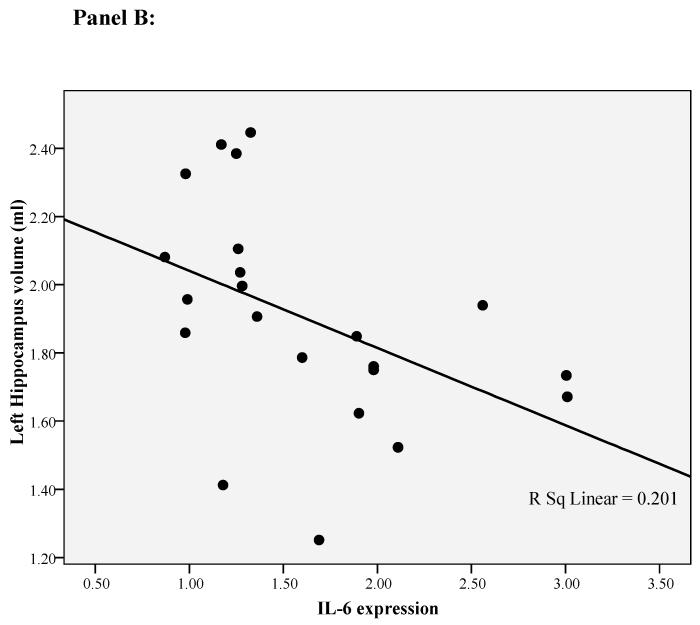
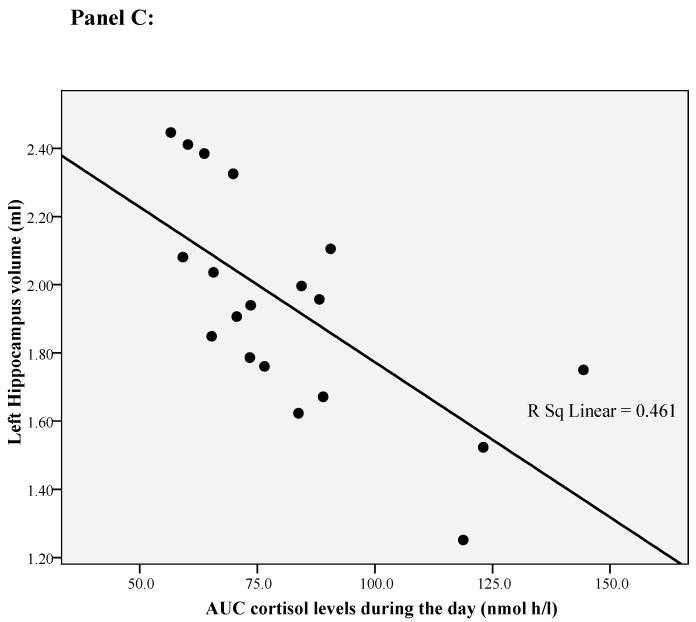
In our previous paper 24 we have found that only the left hippocampal volume is correlated with cortisol levels during the day. This is consistent with studies showing that the left hippocampal volume is exquisitely sensitive to depression and psychosocial stressors 25;26 and that mainly the left-side hippocampal volume is smaller in first episode psychosis 41;42. Therefore, for this paper we ran exploratory correlation analyses between left hippocampal volume and both the immune markers and the psychosocial stress measures. BDNF and IL-6 mRNA levels, and diurnal cortisol levels, all correlated with the left hippocampal volume (respectively r=0.53, p=0.01, r=-0.45, p=0.04, and r=-0.68. p=0.001; see Figure 1 panel A, B and C). None of the other variables was correlated with left hippocampal volume (See Supplemental Material, Table S2).
Figure 1.
Correlation of left hippocampal volume with diurnal cortisol levels, IL-6 gene expression and BDNF gene expression in first-episode psychosis patients. Panel A: Correlation between left hippocampal volume and BDNF gene expression. Panel B: Correlation between left hippocampal volume and IL-6 gene expression. Panel C: Correlation between left hippocampal volume and diurnal cortisol levels.
Multiple Linear Regression Analyses for left hippocampal volume in first-episode psychosis
In order to establish possible predictors of (left) hippocampal volume in patients with first-episode psychosis, we ran a linear regression analysis including all the variables identified statistically to be correlated with this measure. We found that lower BDNF gene expression, higher IL-6 gene expression and higher cortisol levels were independently associated with a smaller left hippocampal volume, and that including one, two or three variables in the models progressively increased the variance explained. The first model of the linear regression included only BDNF gene expression; this accounted for 36% of the variance in left hippocampal volume (adjusted R square, p=0.004, see Figure 1). The second model included BDNF and IL-6 gene expression; this accounted for 47% of the variance in left hippocampal volume (adjusted R square, p=0.002). The third model included BDNF, IL-6 gene expression and diurnal cortisol levels; this accounted for 71% of the variance in left hippocampal volume (adjusted R square, p<0.001). See Table 3.
Table 3.
Multiple Linear Regression for left hippocampal volume in first-episode psychosis patients.
| R | Adjusted R square | Significance | ||
|---|---|---|---|---|
| 1st model | BDNF expression | 0.63 | 0.36 | p=0.004 |
| 2nd model | BDNF expression + IL-6 expression | 0.73 | 0.47 | p=0.002 |
| 3rd model | BDNF expression + IL-6 expression + Diurnal cortisol levels | 0.87 | 0.71 | p<0.001 |
Discussion
Our findings suggest that psychosocial stressors, both during childhood and closer to psychosis onset, play a role in reducing BDNF mRNA levels in leukocytes of first-episode psychosis, possibly through a stress-induced increase in IL-6 expression. In turn, the reduced BDNF expression, together with the increased inflammation and the increased cortisol levels, seem to contribute to the smaller left hippocampal volume in these patients.
Studies in animals have reported that early adverse experiences and chronic stress in adults lead to a persistent reduction of BDNF levels in various areas of the brain 43-45. In contrast, an enriched early social environment has been shown to increase BDNF levels, especially in the hippocampus 46. Interestingly, the very few data available in clinical populations seem to support the notion that psychosocial stress has a negative effect on BDNF levels. Indeed, three studies have found that the number of stressful events, and the severity of childhood neglect, are negatively correlated with peripheral BDNF levels in patients with affective disorders 47-49. Our study is the first to report a negative effect of stressful events, both in childhood and closer to psychosis onset, on BDNF expression in patients with first-episode psychosis. Of note, these effects of psychosocial stressors on BDNF mRNA levels appear stronger in the patients rather than in controls. Although, findings in animal studies show that stress in itself is enough to affect BDNF levels 43-45, the three aforementioned clinical studies examining the effects of psychosocial stressors on BDNF are in samples of patients with depression or bipolar disorder 47-49. Therefore it is possible that psychosis (or, indeed, an affective disorder) creates a greater biological vulnerability of relevant pathways molecular (including BDNF) to stressful experiences. However, patients in our sample also show higher rates of psychosocial stressors. Therefore, the larger number of events is another potential, not mutually exclusive, explanation for the stronger associations between stress and BDNF in patients than in controls.
Interestingly, this is also the first clinical study suggesting that the effects of psychosocial stressors on BDNF expression are mediated by increased levels of inflammation, as shown by the fact that independent effect of IL-6 on BDNF levels disappears once the effects of psychosocial stressors are taken into account. Previous preclinical studies have proposed a number of mechanisms underlying the stress-related down-regulation of BDNF expression, including increased release of pro-inflammatory cytokines 50. Indeed, administration of IL-1 beta has been shown to down-regulate BDNF expression in the rat hippocampus 51;52. The mechanisms through which cytokines might influence BDNF expression are still unclear. Preclinical studies have suggested that pro-inflammatory cytokines could directly reduce BDNF gene transcription via pathways such as cyclic AMP-response element-binding protein phosphorylation or of nuclear factor-κB activation (NF-kB) 53-55.
We also find a correlation between lower BDNF expression and smaller left hippocampal volume in first-episode psychosis. Our findings are consistent with previous studies investigating the effect of BDNF val66met polymorphism on hippocampal volume 56;57. Specifically, these studies found a smaller hippocampal volume in healthy subjects and patients with first-episode schizophrenia carrying the met BDNF allele, which has also been associated with lower depolarization-induced production of BDNF 56;57. However, our data indicate that lower BDNF levels only account for 36% of the variance of hippocampal volume. Adding IL-6 to the model increases the accounted variance to 47%, while the best model predicting hippocampal volume in our patients includes BDNF, IL-6 and cortisol levels, accounting for more than 70% of the variance. This suggests that these three biological pathways independently contribute to the regulation of hippocampal volume. Interestingly BDNF and pro-inflammatory cytokines can act on common intracellular pathways relevant to neuroplasticity, like the mitogen-activated protein (MAP) kinase cascade and the phosphotidylinositol-3 kinase/Akt signalling, and downstream regulators of apoptosis such as Bad (a major pro-apoptotic protein) and bcl-2 (a major anti-apoptotic protein) 58. BDNF and pro-inflammatory cytokines are also well known to exert opposite effect on the regulation of the nuclear translocation of NF-kB, an important transcription factor decreasing cell survival 59;60. Glucocorticoids influence neuroplasticity through their interaction with excitatory amino acid neurotransmitters and NMDA receptors 61. Therefore, our findings suggest a synergic effect of BDNF, IL-6 and cortisol in determining smaller hippocampal volume, possibly through the activation of common molecular pathways.
From a clinical point of view, our findings further support the role of stress in the onset and outcome of psychosis, as already suggested by previous studies. Garner et al., 62 reported a larger pituitary volume in people at high risk of developing psychosis who then went on to develop psychosis within 1 year, suggesting that HPA axis hyperactivity is present already before the onset of psychosis and predicts those who made the transition to psychotic episode. Moreover, a more recent study in drug naïve first-episode psychosis patients found that a larger pituitary volume at onset is associated with less improvement in psychotic symptoms after 12 weeks of antipsychotic treatment, further supporting the role of HPA axis hyperactivity in clinical outcome of these patients 63. Furthermore, studies using glucocorticoid antagonist mifepristone, to target HPA axis activity, have reported clinical improvement in patients with major psychotic depression64-66. More recently, drugs targeting immune system, such as COX-2 inhibitor (celecoxib) or COX-1/COX-2 inhibitor (aspirin), have also been reported to significantly improve psychotic symptoms in patients with schizophrenia spectrum disorders, when used as adjunct therapy to antipsychotics 67-70. Future pharmacological studies should also take into account the relevance of reduced BDNF in psychosis as possible future target for drugs development.
A few limitations need to be acknowledged. First and foremost, our correlation analyses cannot unequivocally imply causation. Therefore, the observed association between high levels of stress and reduced BDNF expression could also have alternative interpretations. For example, through reversed causality, reduced BDNF expression could lead to the increased number of stressors, through an effect on cognitive performance and personality traits in these patients 12;71;72. Similarly, the smaller hippocampal volume may determine the higher cortisol and cytokines levels by reducing negative feedback of the HPA axis and inducing glucocorticoid resistance, a condition associated with inflammation 73. Moreover, our correlation analyses were exploratory and were not corrected for multiple comparisons. Indeed, the large variance of hippocampal volume explained by the three combined stress biomarkers in the linear regression model is surprising, and these findings would need to be confirmed by future studies. A second limitation of this study is represented by our proposition that MRI volumes may represent a measure of neuroplasticity and neuronal function. Indeed, differences in MRI brain volumes might reflect alterations not only in neuronal cells, but also in non-neuronal tissue compartments (e.g., changes in tissue perfusion, fat, and water content) 74. Finally, only a subsample of patients (and none of the controls) accepted to undergo the brain MRI scan.
In conclusion, our findings suggest that the down-regulation of BDNF expression in first-episode psychosis is partly induced by childhood trauma and adulthood stressful events through a biological pathway that may involve increased inflammation. In turn, stress-related biological pathways, together with decreased BDNF expression, may account for the smaller left hippocampal volume. We believe that these biological pathways should be considered for the development of future therapeutic strategies in this condition.
Supplementary Material
Acknowledgments
This research has been supported by the South London and Maudsley NHS Foundation Trust & Institute of Psychiatry NIHR Biomedical Research Centre for Mental Health; a King’s College Development Trust (UK) Studentship, a NARSAD Young Investigator Award, and a grant from the University of London Central Research Fund to V. Mondelli; a KCL Translational Research Grant and a grant from the BIAL Foundation to P. Dazzan; and grants from the British Academy, the UK Medical Research Council, and the Commission of European Communities 7th Framework Programme Collaborative Project Grant Agreement n°22963 (Mood Inflame), to C.M. Pariante; and a grant from the Wellcome Trust (Grant Number: WT087417) to P. Dazzan, C. Morgan, C.M. Pariante and R. Murray.
Footnotes
Financial Disclosure: Nothing to disclose.
References
- 1.Durany N, Michel T, Zochling R, et al. Brain-derived neurotrophic factor and neurotrophin 3 in schizophrenic psychoses. Schizophr Res. 2001;52:79–86. doi: 10.1016/s0920-9964(00)00084-0. [DOI] [PubMed] [Google Scholar]
- 2.Ikeda Y, Yahata N, Ito I, et al. Low serum levels of brain-derived neurotrophic factor and epidermal growth factor in patients with chronic schizophrenia. Schizophr Res. 2008;101:58–66. doi: 10.1016/j.schres.2008.01.017. [DOI] [PubMed] [Google Scholar]
- 3.Rizos EN, Michalopoulou PG, Siafakas N, et al. Association of serum brain-derived neurotrophic factor and duration of untreated psychosis in first-episode patients with schizophrenia. Neuropsychobiology. 2010;62:87–90. doi: 10.1159/000315438. [DOI] [PubMed] [Google Scholar]
- 4.Buckley PF, Pillai A, Evans D, et al. Brain derived neurotropic factor in first-episode psychosis. Schizophr Res. 2007;91:1–5. doi: 10.1016/j.schres.2006.12.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Jindal RD, Pillai AK, Mahadik SP, et al. Decreased BDNF in patients with antipsychotic naive first episode schizophrenia. Schizophr Res. 2010;119:47–51. doi: 10.1016/j.schres.2009.12.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chen DC, Wang J, Wang B, et al. Decreased levels of serum brain-derived neurotrophic factor in drug-naive first-episode schizophrenia: relationship to clinical phenotypes. Psychopharmacology (Berl) 2009;207:375–380. doi: 10.1007/s00213-009-1665-6. [DOI] [PubMed] [Google Scholar]
- 7.Gonzalez-Pintoa A, Mosquera F, Palomino A, et al. Increase in brain-derived neurotrophic factor in first episode psychotic patients after treatment with atypical antipsychotics. Int Clin Psychopharmacol. 2010;25:241–245. doi: 10.1097/yic.0b013e328338bc5a. [DOI] [PubMed] [Google Scholar]
- 8.Lewin GR, Barde YA. Physiology of the neurotrophins. Annu Rev Neurosci. 1996;19:289–317. doi: 10.1146/annurev.ne.19.030196.001445. [DOI] [PubMed] [Google Scholar]
- 9.Koolschijn PC, van Haren NE, Cahn W, et al. Hippocampal volume change in schizophrenia. J Clin Psychiatry. 2010;71:737–744. doi: 10.4088/JCP.08m04574yel. [DOI] [PubMed] [Google Scholar]
- 10.Takahashi T, Wood SJ, Yung AR, et al. Progressive gray matter reduction of the superior temporal gyrus during transition to psychosis. Arch Gen Psychiatry. 2009;66:366–376. doi: 10.1001/archgenpsychiatry.2009.12. [DOI] [PubMed] [Google Scholar]
- 11.Cahn W, Rais M, Stigter FP, et al. Psychosis and brain volume changes during the first five years of schizophrenia. Eur Neuropsychopharmacol. 2009;19:147–151. doi: 10.1016/j.euroneuro.2008.10.006. [DOI] [PubMed] [Google Scholar]
- 12.Hariri AR, Goldberg TE, Mattay VS, et al. Brain-derived neurotrophic factor val66met polymorphism affects human memory-related hippocampal activity and predicts memory performance. J Neurosci. 2003;23:6690–6694. doi: 10.1523/JNEUROSCI.23-17-06690.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Reichenberg A, Harvey PD. Neuropsychological impairments in schizophrenia: Integration of performance-based and brain imaging findings. Psychol Bull. 2007;133:833–858. doi: 10.1037/0033-2909.133.5.833. [DOI] [PubMed] [Google Scholar]
- 14.Aas M, Dazzan P, Mondelli V, et al. Abnormal cortisol awakening response predicts worse cognitive function in patients with first-episode psychosis. Psychol Med. 2010:1–14. doi: 10.1017/S0033291710001170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Murakami S, Imbe H, Morikawa Y, et al. Chronic stress, as well as acute stress, reduces BDNF mRNA expression in the rat hippocampus but less robustly. Neurosci Res. 2005;53:129–139. doi: 10.1016/j.neures.2005.06.008. [DOI] [PubMed] [Google Scholar]
- 16.Schaaf MJ, de Kloet ER, Vreugdenhil E. Corticosterone effects on BDNF expression in the hippocampus. Implications for memory formation. Stress. 2000;3:201–208. doi: 10.3109/10253890009001124. [DOI] [PubMed] [Google Scholar]
- 17.Lapchak PA, Araujo DM, Hefti F. Systemic interleukin-1 beta decreases brain-derived neurotrophic factor messenger RNA expression in the rat hippocampal formation. Neuroscience. 1993;53:297–301. doi: 10.1016/0306-4522(93)90196-m. [DOI] [PubMed] [Google Scholar]
- 18.Sousa N, Madeira MD, Paula-Barbosa MM. Effects of corticosterone treatment and rehabilitation on the hippocampal formation of neonatal and adult rats. An unbiased stereological study. Brain Res. 1998;794:199–210. doi: 10.1016/s0006-8993(98)00218-2. [DOI] [PubMed] [Google Scholar]
- 19.Ekstrand J, Hellsten J, Tingstrom A. Environmental enrichment, exercise and corticosterone affect endothelial cell proliferation in adult rat hippocampus and prefrontal cortex. Neurosci Lett. 2008;442:203–207. doi: 10.1016/j.neulet.2008.06.085. [DOI] [PubMed] [Google Scholar]
- 20.Monje ML, Toda H, Palmer TD. Inflammatory blockade restores adult hippocampal neurogenesis. Science. 2003;302:1760–1765. doi: 10.1126/science.1088417. [DOI] [PubMed] [Google Scholar]
- 21.Marsland AL, Gianaros PJ, Abramowitch SM, et al. Interleukin-6 covaries inversely with hippocampal grey matter volume in middle-aged adults. Biol Psychiatry. 2008;64:484–490. doi: 10.1016/j.biopsych.2008.04.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mondelli V, Dazzan P, Hepgul N, et al. Abnormal cortisol levels during the day and cortisol awakening response in first-episode psychosis: the role of stress and of antipsychotic treatment. Schizophr Res. 2010;116:234–242. doi: 10.1016/j.schres.2009.08.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Potvin S, Stip E, Sepehry AA, et al. Inflammatory cytokine alterations in schizophrenia: a systematic quantitative review. Biol Psychiatry. 2008;63:801–808. doi: 10.1016/j.biopsych.2007.09.024. [DOI] [PubMed] [Google Scholar]
- 24.Mondelli V, Pariante CM, Navari S, et al. Higher cortisol levels are associated with smaller left hippocampal volume in first-episode psychosis. Schizophr Res. 2010;119:75–78. doi: 10.1016/j.schres.2009.12.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Stein MB, Koverola C, Hanna C, et al. Hippocampal volume in women victimized by childhood sexual abuse. Psychol Med. 1997;27:951–959. doi: 10.1017/s0033291797005242. [DOI] [PubMed] [Google Scholar]
- 26.Vythilingam M, Heim C, Newport J, et al. Childhood trauma associated with smaller hippocampal volume in women with major depression. Am J Psychiatry. 2002;159:2072–2080. doi: 10.1176/appi.ajp.159.12.2072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Karege F, Schwald M, Cisse M. Postnatal developmental profile of brain-derived neurotrophic factor in rat brain and platelets. Neurosci Lett. 2002;328:261–264. doi: 10.1016/s0304-3940(02)00529-3. [DOI] [PubMed] [Google Scholar]
- 28.Klein AB, Williamson R, Santini MA, et al. Blood BDNF concentrations reflect brain-tissue BDNF levels across species. Int J Neuropsychopharmacol. 2010:1–7. doi: 10.1017/S1461145710000738. [DOI] [PubMed] [Google Scholar]
- 29.Sullivan PF, Fan C, Perou CM. Evaluating the comparability of gene expression in blood and brain. Am J Med Genet B Neuropsychiatr Genet. 2006;141B:261–268. doi: 10.1002/ajmg.b.30272. [DOI] [PubMed] [Google Scholar]
- 30.Pillai A, Kale A, Joshi S, et al. Decreased BDNF levels in CSF of drug-naive first-episode psychotic subjects: correlation with plasma BDNF and psychopathology. Int J Neuropsychopharmacol. 2010;13:535–539. doi: 10.1017/S1461145709991015. [DOI] [PubMed] [Google Scholar]
- 31.Glatt SJ, Everall IP, Kremen WS, et al. Comparative gene expression analysis of blood and brain provides concurrent validation of SELENBP1 up-regulation in schizophrenia. Proc Natl Acad Sci U S A. 2005;102:15533–15538. doi: 10.1073/pnas.0507666102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Cattaneo A, Bocchio-Chiavetto L, Zanardini R, et al. Reduced peripheral brain-derived neurotrophic factor mRNA levels are normalized by antidepressant treatment. Int J Neuropsychopharmacol. 2010;13:103–108. doi: 10.1017/S1461145709990812. [DOI] [PubMed] [Google Scholar]
- 33.World Health Organisation . The ICD-10 Classification of Mental and Behavioural Disorders: clinical descriptions and diagnostic guidelines. Geneva: 1992. [Google Scholar]
- 34.Bebbington P, Nayani T. The psychosis screening questionnaire. International Journal of Methods in Psychiatric Research. 1995;5:11–19. [Google Scholar]
- 35.McGuffin P, Farmer A, Harvey I. A polydiagnostic application of operational criteria in studies of psychotic illness. Development and reliability of the OPCRIT system. Arch Gen Psychiatry. 1991;48:764–770. doi: 10.1001/archpsyc.1991.01810320088015. [DOI] [PubMed] [Google Scholar]
- 36.Brugha TS, Cragg D. The List of Threatening Experiences: the reliability and validity of a brief life events questionnaire. Acta Psychiatr Scand. 1990;82:77–81. doi: 10.1111/j.1600-0447.1990.tb01360.x. [DOI] [PubMed] [Google Scholar]
- 37.Cohen S, Williamson G. Perceived stress in a probability sample of the United Stated. In: Spacapan S, Oskamp S, editors. The social psychology of health: Claremont Symposium on applied social psychology. Newbury Park, CA: 1988. [Google Scholar]
- 38.Bifulco A, Bernazzani O, Moran PM, et al. The childhood experience of care and abuse questionnaire (CECA.Q): validation in a community series. Br J Clin Psychol. 2005;44:563–581. doi: 10.1348/014466505X35344. [DOI] [PubMed] [Google Scholar]
- 39.Pruessner JC, Kirschbaum C, Meinlschmid G, et al. Two formulas for computation of the area under the curve represent measures of total hormone concentration versus time-dependent change. Psychoneuroendocrinology. 2003;28:916–931. doi: 10.1016/s0306-4530(02)00108-7. [DOI] [PubMed] [Google Scholar]
- 40.Schulze K, McDonald C, Frangou S, et al. Hippocampal volume in familial and nonfamilial schizophrenic probands and their unaffected relatives. Biol Psychiatry. 2003;53:562–570. doi: 10.1016/s0006-3223(02)01910-8. [DOI] [PubMed] [Google Scholar]
- 41.Steen RG, Mull C, McClure R, et al. Brain volume in first-episode schizophrenia: systematic review and meta-analysis of magnetic resonance imaging studies. Br J Psychiatry. 2006;188:510–518. doi: 10.1192/bjp.188.6.510. [DOI] [PubMed] [Google Scholar]
- 42.Velakoulis D, Wood SJ, Wong MT, et al. Hippocampal and amygdala volumes according to psychosis stage and diagnosis: a magnetic resonance imaging study of chronic schizophrenia, first-episode psychosis, and ultra-high-risk individuals. Arch Gen Psychiatry. 2006;63:139–149. doi: 10.1001/archpsyc.63.2.139. [DOI] [PubMed] [Google Scholar]
- 43.Roceri M, Cirulli F, Pessina C, et al. Postnatal repeated maternal deprivation produces age-dependent changes of brain-derived neurotrophic factor expression in selected rat brain regions. Biol Psychiatry. 2004;55:708–714. doi: 10.1016/j.biopsych.2003.12.011. [DOI] [PubMed] [Google Scholar]
- 44.Duman RS, Monteggia LM. A neurotrophic model for stress-related mood disorders. Biol Psychiatry. 2006;59:1116–1127. doi: 10.1016/j.biopsych.2006.02.013. [DOI] [PubMed] [Google Scholar]
- 45.Roth TL, Lubin FD, Funk AJ, et al. Lasting epigenetic influence of early-life adversity on the BDNF gene. Biol Psychiatry. 2009;65:760–769. doi: 10.1016/j.biopsych.2008.11.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Branchi I, D’Andrea I, Fiore M, et al. Early social enrichment shapes social behavior and nerve growth factor and brain-derived neurotrophic factor levels in the adult mouse brain. Biol Psychiatry. 2006;60:690–696. doi: 10.1016/j.biopsych.2006.01.005. [DOI] [PubMed] [Google Scholar]
- 47.Grassi-Oliveira R, Stein LM, Lopes RP, et al. Low plasma brain-derived neurotrophic factor and childhood physical neglect are associated with verbal memory impairment in major depression--a preliminary report. Biol Psychiatry. 2008;64:281–285. doi: 10.1016/j.biopsych.2008.02.023. [DOI] [PubMed] [Google Scholar]
- 48.Kauer-Sant’anna M, Tramontina J, Andreazza AC, et al. Traumatic life events in bipolar disorder: impact on BDNF levels and psychopathology. Bipolar Disord. 2007;9(Suppl 1):128–135. doi: 10.1111/j.1399-5618.2007.00478.x. [DOI] [PubMed] [Google Scholar]
- 49.Elzinga BM, Molendijk ML, Oude Voshaar RC, et al. The impact of childhood abuse and recent stress on serum brain-derived neurotrophic factor and the moderating role of BDNF Val(66)Met. Psychopharmacology (Berl) 2010 doi: 10.1007/s00213-010-1961-1. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Hayley S, Poulter MO, Merali Z, et al. The pathogenesis of clinical depression: stressor- and cytokine-induced alterations of neuroplasticity. Neuroscience. 2005;135:659–678. doi: 10.1016/j.neuroscience.2005.03.051. [DOI] [PubMed] [Google Scholar]
- 51.Barbany G, Persson H. Regulation of Neurotrophin mRNA Expression in the Rat Brain by Glucocorticoids. Eur J Neurosci. 1992;4:396–403. doi: 10.1111/j.1460-9568.1992.tb00888.x. [DOI] [PubMed] [Google Scholar]
- 52.Barrientos RM, Sprunger DB, Campeau S, et al. Brain-derived neurotrophic factor mRNA downregulation produced by social isolation is blocked by intrahippocampal interleukin-1 receptor antagonist. Neuroscience. 2003;121:847–853. doi: 10.1016/s0306-4522(03)00564-5. [DOI] [PubMed] [Google Scholar]
- 53.Lahiri T, Moore PE, Baraldo S, et al. Effect of IL-1beta on CRE-dependent gene expression in human airway smooth muscle cells. Am J Physiol Lung Cell Mol Physiol. 2002;283:L1239–L1246. doi: 10.1152/ajplung.00231.2001. [DOI] [PubMed] [Google Scholar]
- 54.Parry GC, Mackman N. Role of cyclic AMP response element-binding protein in cyclic AMP inhibition of NF-kappaB-mediated transcription. J Immunol. 1997;159:5450–5456. [PubMed] [Google Scholar]
- 55.Murphy PG, Borthwick LA, Altares M, et al. Reciprocal actions of interleukin-6 and brain-derived neurotrophic factor on rat and mouse primary sensory neurons. Eur J Neurosci. 2000;12:1891–1899. doi: 10.1046/j.1460-9568.2000.00074.x. [DOI] [PubMed] [Google Scholar]
- 56.Bueller JA, Aftab M, Sen S, et al. BDNF Val66Met allele is associated with reduced hippocampal volume in healthy subjects. Biol Psychiatry. 2006;59:812–815. doi: 10.1016/j.biopsych.2005.09.022. [DOI] [PubMed] [Google Scholar]
- 57.Szeszko PR, Lipsky R, Mentschel C, et al. Brain-derived neurotrophic factor val66met polymorphism and volume of the hippocampal formation. Mol Psychiatry. 2005;10:631–636. doi: 10.1038/sj.mp.4001656. [DOI] [PubMed] [Google Scholar]
- 58.Manji HK, Chen G. PKC, MAP kinases and the bcl-2 family of proteins as long-term targets for mood stabilizers. Mol Psychiatry. 2002;7(Suppl 1):S46–S56. doi: 10.1038/sj.mp.4001018. [DOI] [PubMed] [Google Scholar]
- 59.Koo JW, Russo SJ, Ferguson D, et al. Nuclear factor-kappaB is a critical mediator of stress-impaired neurogenesis and depressive behavior. Proc Natl Acad Sci U S A. 2010;107:2669–2674. doi: 10.1073/pnas.0910658107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Brietzke E, Kapczinski F. TNF-alpha as a molecular target in bipolar disorder. Prog Neuropsychopharmacol Biol Psychiatry. 2008;32:1355–1361. doi: 10.1016/j.pnpbp.2008.01.006. [DOI] [PubMed] [Google Scholar]
- 61.McEwen BS. The neurobiology of stress: from serendipity to clinical relevance. Brain Res. 2000;886:172–189. doi: 10.1016/s0006-8993(00)02950-4. [DOI] [PubMed] [Google Scholar]
- 62.Garner B, Pariante CM, Wood SJ, et al. Pituitary volume predicts future transition to psychosis in individuals at ultra-high risk of developing psychosis. Biol Psychiatry. 2005;58:417–423. doi: 10.1016/j.biopsych.2005.04.018. [DOI] [PubMed] [Google Scholar]
- 63.Garner B, Berger GE, Nicolo JP, et al. Pituitary volume and early treatment response in drug-naive first-episode psychosis patients. Schizophr Res. 2009;113:65–71. doi: 10.1016/j.schres.2009.05.008. [DOI] [PubMed] [Google Scholar]
- 64.Belanoff JK, Flores BH, Kalezhan M, et al. Rapid reversal of psychotic depression using mifepristone. J Clin Psychopharmacol. 2001;21:516–521. doi: 10.1097/00004714-200110000-00009. [DOI] [PubMed] [Google Scholar]
- 65.Simpson GM, El SA, Loza N, et al. An 8-week open-label trial of a 6-day course of mifepristone for the treatment of psychotic depression. J Clin Psychiatry. 2005;66:598–602. doi: 10.4088/jcp.v66n0509. [DOI] [PubMed] [Google Scholar]
- 66.Debattista C, Belanoff J. The use of mifepristone in the treatment of neuropsychiatric disorders. Trends Endocrinol Metab. 2006;17:117–121. doi: 10.1016/j.tem.2006.02.006. [DOI] [PubMed] [Google Scholar]
- 67.Muller N, Riedel M, Scheppach C, et al. Beneficial antipsychotic effects of celecoxib add-on therapy compared to risperidone alone in schizophrenia. Am J Psychiatry. 2002;159:1029–1034. doi: 10.1176/appi.ajp.159.6.1029. [DOI] [PubMed] [Google Scholar]
- 68.Muller N, Krause D, Dehning S, et al. Celecoxib treatment in an early stage of schizophrenia: results of a randomized, double-blind, placebo-controlled trial of celecoxib augmentation of amisulpride treatment. Schizophr Res. 2010;121:118–124. doi: 10.1016/j.schres.2010.04.015. [DOI] [PubMed] [Google Scholar]
- 69.Akhondzadeh S, Tabatabaee M, Amini H, et al. Celecoxib as adjunctive therapy in schizophrenia: a double-blind, randomized and placebo-controlled trial. Schizophr Res. 2007;90:179–185. doi: 10.1016/j.schres.2006.11.016. [DOI] [PubMed] [Google Scholar]
- 70.Laan W, Grobbee DE, Selten JP, et al. Adjuvant aspirin therapy reduces symptoms of schizophrenia spectrum disorders: results from a randomized, double-blind, placebo-controlled trial. J Clin Psychiatry. 2010;71:520–527. doi: 10.4088/JCP.09m05117yel. [DOI] [PubMed] [Google Scholar]
- 71.Egan MF, Kojima M, Callicott JH, et al. The BDNF val66met polymorphism affects activity-dependent secretion of BDNF and human memory and hippocampal function. Cell. 2003;112:257–269. doi: 10.1016/s0092-8674(03)00035-7. [DOI] [PubMed] [Google Scholar]
- 72.Jiang X, Xu K, Hoberman J, et al. BDNF variation and mood disorders: a novel functional promoter polymorphism and Val66Met are associated with anxiety but have opposing effects. Neuropsychopharmacology. 2005;30:1353–1361. doi: 10.1038/sj.npp.1300703. [DOI] [PubMed] [Google Scholar]
- 73.Pace TW, Miller AH. Cytokines and glucocorticoid receptor signaling. Relevance to major depression. Ann N Y Acad Sci. 2009;1179:86–105. doi: 10.1111/j.1749-6632.2009.04984.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Weinberger DR, McClure RK. Neurotoxicity, neuroplasticity, and magnetic resonance imaging morphometry: what is happening in the schizophrenic brain? Arch Gen Psychiatry. 2002;59:553–558. doi: 10.1001/archpsyc.59.6.553. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.