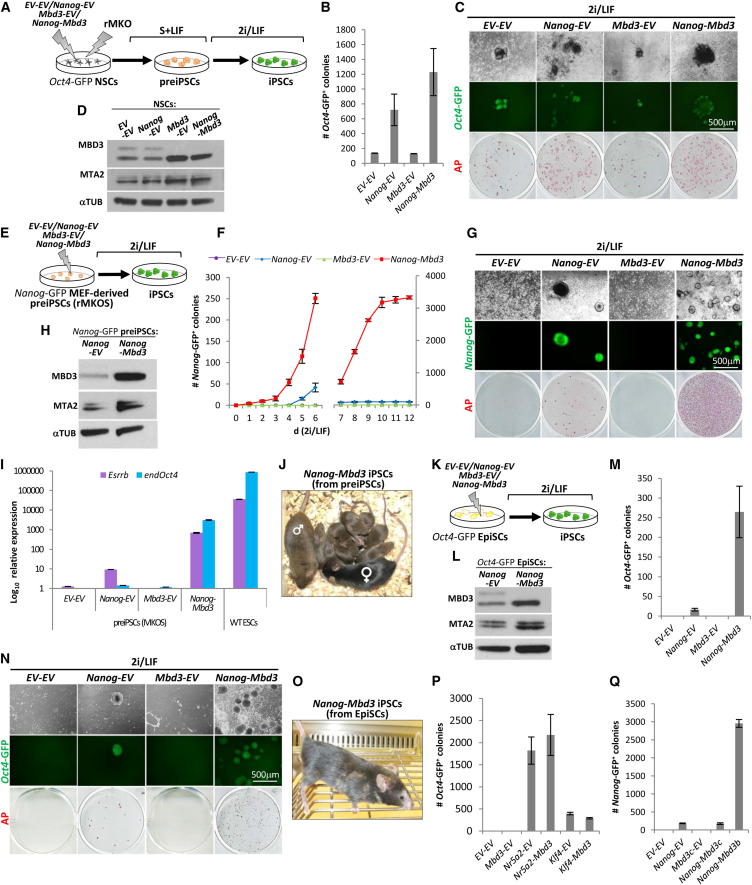
Figure 4.
Overexpression of MBD3/NuRD Facilitates NANOG-Mediated Reprogramming
(A) Experimental design used to address the effect of MBD3 overexpression on NSC reprogramming. NSCs carrying an Oct4-GFP cassette were stably transfected with pPB-CAG-Nanog and pPB-CAG-Mbd3b or pPB-CAG-empty controls, transduced with rOKM, cultured in Egf+Fgf2 medium for 3 days, switched to S+LIF medium for 6 days, and then switched to 2i/LIF conditions.
(B) Quantification of Oct4-GFP+ colonies after 12 days in 2i/LIF conditions. Colony number is per 1.0 × 105 NSCs.
(C) Phase and GFP images and AP staining of the iPSCs obtained from NSCs overexpressing respective transgenes.
(D) Western blot analysis of MBD3, MTA2, and TUBULIN (TUB) protein expression in NSCs overexpressing the indicated transgene combinations.
(E) Experimental design used to address the effect of MBD3/NuRD overexpression on the conversion of preiPSCs to iPSCs. PreiPSCs (carrying a Nanog-GFP) were stably transfected with the same transgene combinations as in (A) and plated in 2i/LIF conditions for 12 days.
(F) The kinetics of the emergence of Nanog-GFP+ colonies from the transgenic preiPSCs during a 12 day culture in 2i/LIF conditions (y axis scale changes at day 7). Colony number is per 1.0 × 105 preiPSCs.
(G) Phase and GFP images and AP staining of the iPSCs formed from preiPSCs overexpressing respective transgenes.
(H) Western blot analysis of MBD3, MTA2, and TUBULIN (TUB) protein expression in preiPSCs overexpressing NANOG or NANOG and MBD3.
(I) qRT-PCR analysis of Esrrβ and endogenous (end) Oct4 expression in preiPSCs 12 days after stable transgene transfection and culture in S+LIF (y axes in log10 scale). The expression levels of Esrrβ and endogenous Oct4 in these Nanog-Mbd3 preiPSCs are 5% and 3%, respectively, of the expression levels of WT ESCs in 2i/LIF. The Esrrβ expression level is also approximately 80 times greater than that of Nanog-EV preiPSCs and 700 times greater than that of Mbd3-EV and EV-EV preiPSCs. The endogenous Oct4 expression level is approximately 2,000 times greater than that of Nanog-EV preiPSCs and 3,000 times greater than that of Mbd3-EV and EV-EV preiPSCs. qRT-PCR values are normalized to Gapdh value and shown as relative to the highest value.
(J) Germ line contribution of Nanog-Mbd3 iPSCs generated from preiPSCs (brown color). Cells were treated with TAT-Cre for reprogramming transgene excision prior to blastocyst injection. Chimeric father, C57BL/6 mother, and pups resulting from cross can be viewed.
(K) Experimental design used to address the effect of MBD3/NuRD overexpression on EpiSC reprogramming. EpiSCs (carrying an Oct4-GFP) were stably transfected with the same transgene combinations as in (A) and (E) and plated in 2i/LIF conditions for 12 days.
(L) Western blot analysis of MBD3, MTA2, and TUBULIN (TUB) protein expression in EpiSCs overexpressing NANOG or NANOG and MBD3.
(M) Quantification of Oct4-GFP+ colonies after 12 days of 2i/LIF culture. Colony numbers are per 2.0 × 104 EpiSCs.
(N) Phase and GFP images and AP staining of the iPSCs formed from EpiSCs overexpressing respective transgenes.
(O) Chimera of Nanog-Mbd3 iPSCs generated from EpiSCs (brown color). Cells were treated with TAT-Cre for reprogramming transgene excision prior to blastocyst injection.
(P) Quantification of Oct4-GFP+ colonies after 12 days of 2i/LIF culture, generated from EpiSCs transfected with Klf4 or Nr5a2 (together or not with Mbd3). Colony numbers are per 2.0 × 104 EpiSCs.
(Q) Quantification of Nanog-GFP+ colonies after 12 days of 2i/LIF culture generated from MEF-derived preiPSCs stably transfected with Nanog alone, or Nanog together with Mbd3b or Mbd3c. Colony numbers are per 1.0 × 105 preiPSCs. The error bars indicate STDEV.