Fig. 3. Possible modulation of NF-kB pathway by EGCG.
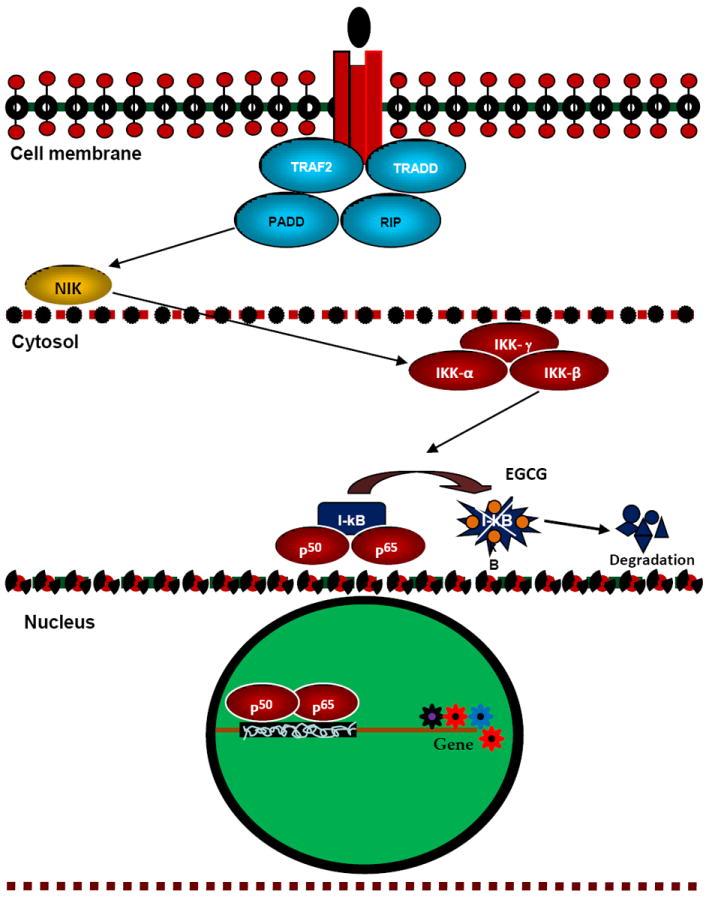
In the cytosol as a result of the binding of p50 and p65 to I-kB, NF-kB becomes inactive. When I-kB is phosphorylated by IKKs and degraded in a proteasome-dependent pathway, p50 and p65 are set free and are translocated into the nucleus to activate a specific set of genes. In vitro and in vivo this pathway has been shown to be inhibited by EGCG both, possibly by inhibiting IKK-catalyzed phosphorylation of I-kB.
