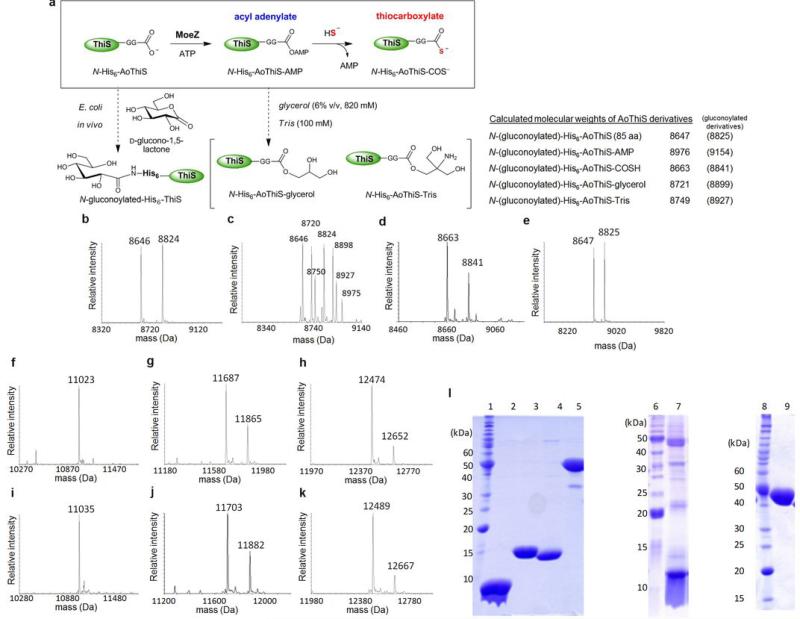
Extended Data Fig. 3. EIS-MS analyses of the AoMoeZ-catalyzed activation of sulphur-carrier proteins and SDS-PAGE of the purified proteins.
a, Reaction scheme of the AoMoeZ-catalyzed activation of AoThiS. b–e, Deconvoluted ESI-MS of as-isolated (b) AoThiS, (c) AoThiS in the presence of AoMoeZ and ATP, (d) AoThiS in the presence of AoMoeZ, ATP, and bisulphide, and (e) AoThiS in the presence of bisulphide (control). The calculated molecular masses are shown as the neutral form in the upper right corner. Analysis of purified N-His6-AoThiS shows two mass signals (obsd, 8646/8824 Da) consistent with the calculated molecular mass of the recombinant enzyme in its native and N-gluconoylated form (calcd, 8647/8825 Da). Gluconoylation of the N-terminal His6-tag is a known post-translational modification when expressing recombinant proteins in E. coli37. Such a modification should not affect AoThiS activity, because the predicted active site for AoThiS is at the C-terminus. Indeed, when N-His6-AoThiS was incubated with N-His6-AoMoeZ and ATP, a MS signal corresponding to the adenylated N-His6-AoThiS (9) was detected along with few peaks likely derived from reaction of the labile adenylated AoThiS with buffer components (see panel c). f–k, Deconvoluted ESI-MS of (f) as-isolated AoMoaD (the calculated molecular mass of N-His6-AoMoaD (105 aa) is 11022 Da), (g) as-isolated AoCysO (the calculated molecular masses of N-His6-AoCysO (109 aa) and its N-gluconoylated derivative are 11688 and 11866, respectively), (h) as-isolated AoMoaD2 (the calculated molecular masses of N-His6-AoMoaD2 (115 aa) and its N-gluconoylated derivative are 12473 and 12651, respectively), (i) AoMoaD incubated with AoMoeZ, ATP, and NaSH (the calculated molecular mass of N-His6-AoMoaD-COSH is 11038 Da), (j) AoCysO incubated with AoMoeZ, ATP, and NaSH (the calculated molecular masses of NHis6-AoCysO-COSH and its N-gluconoylated derivative are 11704 and 11882, respectively), and (k) AoMoaD2 incubated with AoMoeZ, ATP, and NaSH (the calculated molecular masses of N-His6-AoMoaD-COSH and its N-gluconoylated derivative are 12489 and 12667, respectively). l, SDS-PAGE gel of purified sulphur-carrier proteins, AoMoeZ and CD4. NHis6-AoThiS (85 aa, 8.7 kDa, lane 2), N-His6-AoMoaD2 (115 aa, 12.5 kDa, lane 3), N-His6-AoCysO (109 aa, 11.7 kDa, lane 4), N-His6-AoMoeZ (421 aa, 45.0 kDa, lane 5), N-His6-AoMoaD (105 aa, 11.0 kDa, lane 7), and N-His6-CD4 (417 aa, 43.3 kDa, lane 9). The molecular weight marks are 220, 160, 120, 100, 90, 80, 70, 60, 50, 40, 30, 25, 20, 15, and 10 kDa (top to bottom, lane 1, 6 and 8). The protein AoMoaD was not expressed well, and the partially purified protein solution contained significant amounts of endogenous proteins from the E. coli host.