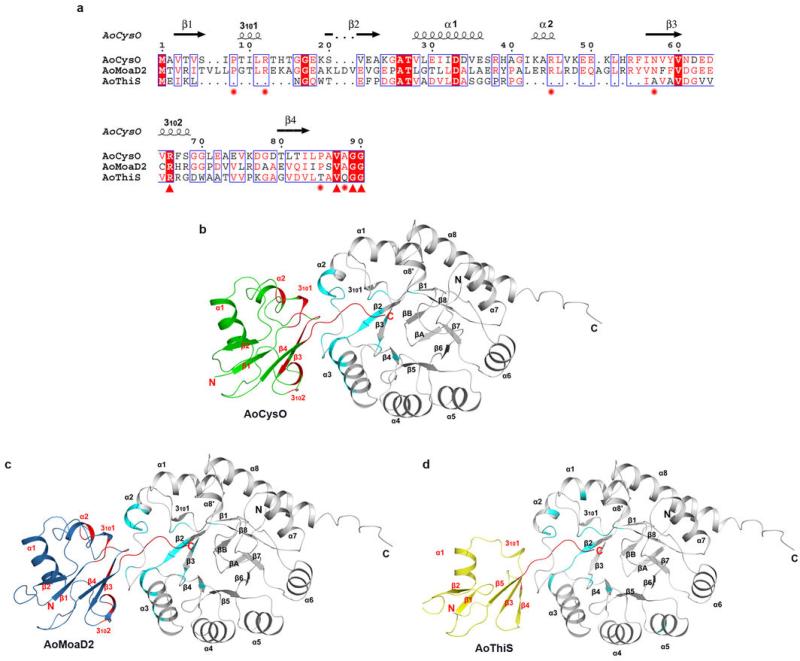
Extended Data Fig. 5. Sequence alignment of AoCysO, AoMoaD2 and AoThiS and hydrophobic interactions for BexX complexes.
a, Sequence alignment was performed based on structural supersession using the programs 3D-Coffee38, MultAlin39 and ESPript40. Main differences between AoCysO (or AoMoaD2) and AoThiS result from an insertion of ten residues between β1 and β2 of AoCysO (or AoMoaD2) and an insertion of 14 (or 15) residues between α1 and β3 of AoCysO (or AoMoaD2). The first insertion includes the short helix 3101 and the second includes helix α2. Both of these insertions are involved in the BexX-AoCysO (or BexX-AoMoaD2) interface. Ten interface residues (marked with red star and red triangle) are conserved between AoCysO and AoMoaD2, however, only four residues are conserved in AoThiS (marked with red triangle). Two differences between AoCysO and AoMoaD2 represent conservative substitutions; while Thr9 and Ala86 in AoCysO is replaced by Gly11 and Ser92 in AoMoaD2, the interface interaction is contributed by hydrogen bonds that formed by the backbone atom. b–d, Hydrophobic interactions of (b) BexX/AoCysO, (c) BexX/AoMoaD2, and (d) BexX/AoThiS. BexX monomers are shown as gray ribbon diagrams with hydrophobic interaction regions colored in cyan. AoCysO, AoMoaD2 and AoThiS are shown as cartoon and colored in green, skyblue and yellow, respectively. Hydrophobic interaction regions in sulphur carrier protein are colored in red. The α-helices and β-strands in BexX and sulphur carrier proteins are labeled in black and red, respectively.