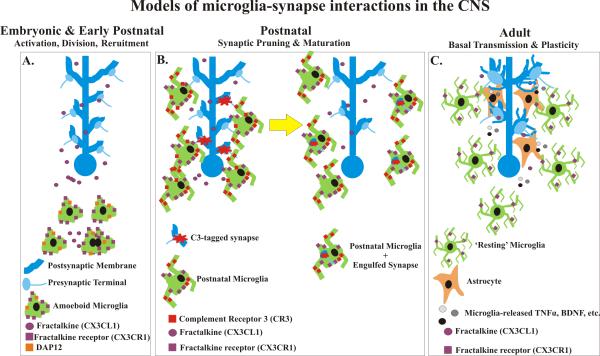
Figure 1. Models of microglia-synapse interactions in the CNS.
A. In the embryonic and early postnatal brain, microglia are of an amoeboid morphology resembling ‘activated’ cells associated with disease and injury. During this stage, they are actively dividing and recruited to regions throughout the CNS. Fractalkine (purple circles), which may be released by neurons, is proposed to act on fractalkine receptors (purple squares) expressed by microglia to regulate activation, cell number, and/or recruitment to synaptic-enriched regions. DAP12 (orange squares) expressed on the surface of microglia is also thought to affect synapse function during this period, perhaps, by regulating the ‘activation’ state of microglia. B. In the postnatal brain, during the first 3 weeks of postnatal life, microglia, which are still ‘activated’ but now have processes, participate in synaptic pruning. Along with the fractalkine receptor (purple squares) and DAP12 (orange squares), microglia express high levels of complement receptor 3 (CR3, red squares) on their surface. We propose that complement component C3 (red stars) is tagging synapses for removal and have demonstrated that synapses are engulfed by phagocytic microglia in a complement-dependent manner. Disruptions in any of these processes results in deficits in synaptic pruning or maturation. C. In the adult brain, evidence suggests that microglia are releasing soluble factors (grey and black circles) such as BDNF, TNFα, glycine, L-serine etc., which affect basal neurotransmission and synaptic plasticity (i.e., LTP) via direct action on neurons or indirectly via astrocytes (orange). In addition, fractalkine signaling via soluble fractalkine (purple circles), most likely released by neurons, and the fractalkine receptor (purple squares), expressed by microglia, modulates microglia-synapse interactions to affect LTP and behavior in the mature CNS.