Abstract
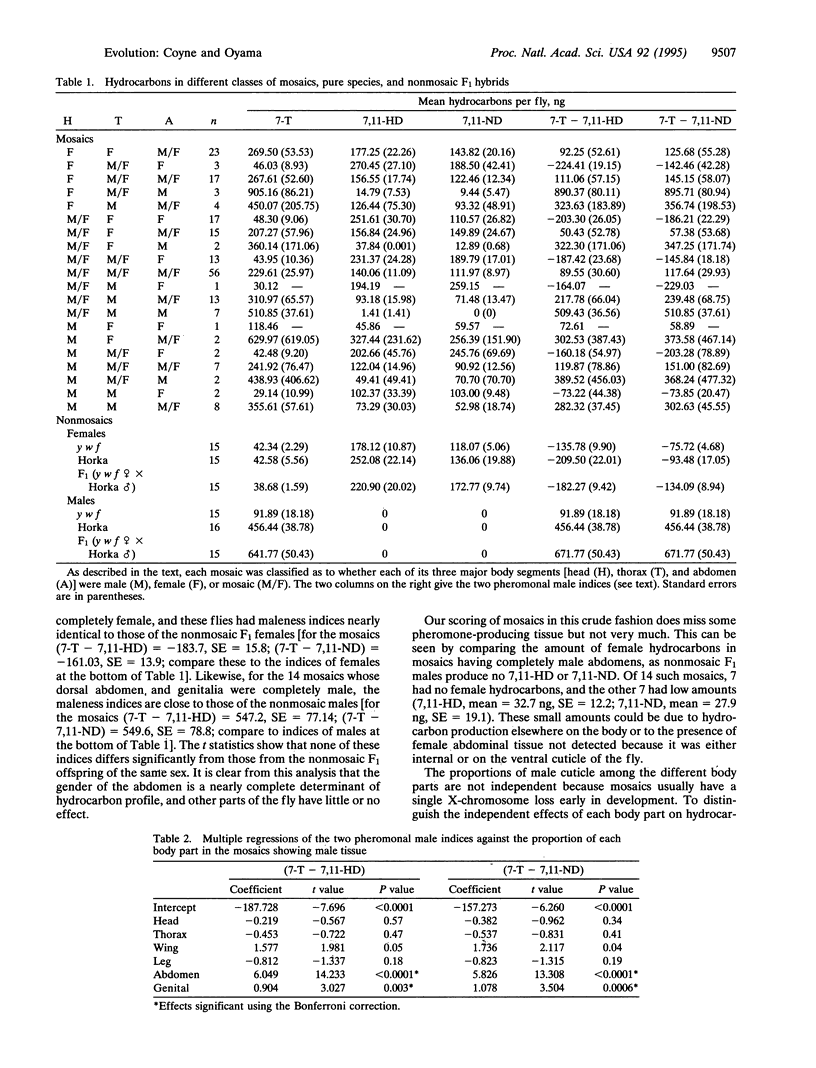
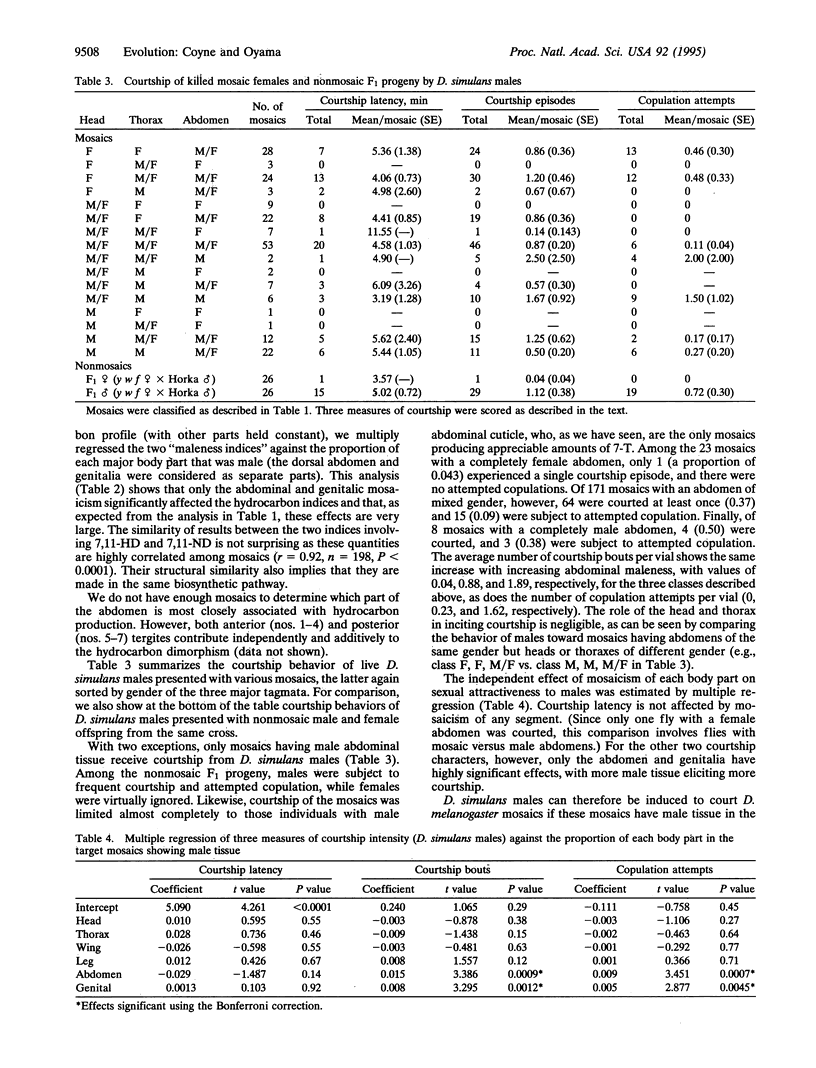
Drosophila melanogaster is sexually dimorphic for cuticular hydrocarbons, with males and females having strikingly different profiles of the long-chain compounds that act as contact pheromones. Gas-chromatographic analysis of sexual mosaics reveals that the sex specificity of hydrocarbons is located in the abdomen. This explains previous observations that D. melanogaster males display the strongest courtship toward mosaics with female abdomens. We also show that males of the sibling species Drosophila simulans preferentially court D. melanogaster mosaics with male abdomens. Because the primary male hydrocarbon in D. melanogaster is also the primary female hydrocarbon in D. simulans, this supports the idea that interspecific differences in cuticular hydrocarbons contribute to sexual isolation.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Carlson D. A., Mayer M. S., Silhacek D. L., James J. D., Beroza M., Bierl B. A. Sex attractant pheromone of the house fly: isolation, identification and synthesis. Science. 1971 Oct 1;174(4004):76–78. doi: 10.1126/science.174.4004.76. [DOI] [PubMed] [Google Scholar]
- Coyne J. A., Crittenden A. P., Mah K. Genetics of a pheromonal difference contributing to reproductive isolation in Drosophila. Science. 1994 Sep 2;265(5177):1461–1464. doi: 10.1126/science.8073292. [DOI] [PubMed] [Google Scholar]
- Coyne J. A. Genetics of sexual isolation between two sibling species, Drosophila simulans and Drosophila mauritiana. Proc Natl Acad Sci U S A. 1989 Jul;86(14):5464–5468. doi: 10.1073/pnas.86.14.5464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coyne J. A. Genetics of sexual isolation in females of the Drosophila simulans species complex. Genet Res. 1992 Aug;60(1):25–31. doi: 10.1017/s0016672300030639. [DOI] [PubMed] [Google Scholar]
- Ferveur J. F. Genetic control of pheromones in Drosophila simulans. I. Ngbo, a locus on the second chromosome. Genetics. 1991 Jun;128(2):293–301. doi: 10.1093/genetics/128.2.293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferveur J. F., Jallon J. M. Genetic control of pheromones in Drosophila simulans. II. kété, a locus on the X chromosome. Genetics. 1993 Mar;133(3):561–567. doi: 10.1093/genetics/133.3.561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferveur J. F., Störtkuhl K. F., Stocker R. F., Greenspan R. J. Genetic feminization of brain structures and changed sexual orientation in male Drosophila. Science. 1995 Feb 10;267(5199):902–905. doi: 10.1126/science.7846534. [DOI] [PubMed] [Google Scholar]
- Hall J. C. Portions of the central nervous system controlling reproductive behavior in Drosophila melanogaster. Behav Genet. 1977 Jul;7(4):291–312. doi: 10.1007/BF01066800. [DOI] [PubMed] [Google Scholar]
- Jallon J. M. A few chemical words exchanged by Drosophila during courtship and mating. Behav Genet. 1984 Sep;14(5):441–478. doi: 10.1007/BF01065444. [DOI] [PubMed] [Google Scholar]
- Jallon J. M., Hotta Y. Genetic and behavioral studies of female sex appeal in Drosophila. Behav Genet. 1979 Jul;9(4):257–275. doi: 10.1007/BF01068205. [DOI] [PubMed] [Google Scholar]
- Pechine J. M., Perez F., Antony C., Jallon J. M. A further characterization of Drosophila cuticular monoenes using a mass spectrometry method to localize double bonds in complex mixtures. Anal Biochem. 1985 Feb 15;145(1):177–182. doi: 10.1016/0003-2697(85)90344-6. [DOI] [PubMed] [Google Scholar]
- Roelofs W., Glover T., Tang X. H., Sreng I., Robbins P., Eckenrode C., Löfstedt C., Hansson B. S., Bengtsson B. O. Sex pheromone production and perception in European corn borer moths is determined by both autosomal and sex-linked genes. Proc Natl Acad Sci U S A. 1987 Nov;84(21):7585–7589. doi: 10.1073/pnas.84.21.7585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szabad J., Máthé E., Puro J. Horka, a dominant mutation of Drosophila, induces nondisjunction and, through paternal effect, chromosome loss and genetic mosaics. Genetics. 1995 Apr;139(4):1585–1599. doi: 10.1093/genetics/139.4.1585. [DOI] [PMC free article] [PubMed] [Google Scholar]


