Abstract
Background
We aimed to assess the efficacy of the smartphone-based health application for glucose control and patient satisfaction with the mobile network system used for glucose self-monitoring.
Methods
Thirty-five patients were provided with a smartphone device, and self-measured blood glucose data were automatically transferred to the medical staff through the smartphone application over the course of 12 weeks. The smartphone user group was divided into two subgroups (more satisfied group vs. less satisfied group) based on the results of questionnaire surveys regarding satisfaction, comfort, convenience, and functionality, as well as their willingness to use the smartphone application in the future. The control group was set up via a review of electronic medical records by group matching in terms of age, sex, doctor in charge, and glycated hemoglobin (HbA1c).
Results
Both the smartphone group and the control group showed a tendency towards a decrease in the HbA1c level after 3 months (7.7%±0.7% to 7.5%±0.7%, P=0.077). In the more satisfied group (n=27), the HbA1c level decreased from 7.7%±0.8% to 7.3%±0.6% (P=0.001), whereas in the less satisfied group (n=8), the HbA1c result increased from 7.7%±0.4% to 8.1%±0.5% (P=0.062), showing values much worse than that of the no-smartphone control group (from 7.7%±0.5% to 7.7%±0.7%, P=0.093).
Conclusion
In addition to medical feedback, device and network-related patient satisfaction play a crucial role in blood glucose management. Therefore, for the smartphone app-based blood glucose monitoring to be effective, it is essential to provide the patient with a well-functioning high quality tool capable of increasing patient satisfaction and willingness to use.
Keywords: Delivery of health care, Diabetes mellitus, Information technology, Smartphone, Ubiquitous
INTRODUCTION
Recently, public healthcare expenditures have been increasing exponentially due to a plethora of chronic diseases such as diabetes and the aging of the population [1,2,3]. Despite the continuing development of pharmaceuticals and therapeutic measures, this tendency appears to be increasing with time. Thus, we are at point where a new type of chronic disease management system should be established. It is against this backdrop that the Ubiquitous-Healthcare (U-Healthcare) has come into existence. The recently commercialized U-Healthcare makes health management possible without temporal and geographical limits by facilitating doctor-patient communication over the internet or via cellular phones [4,5]. This technology is mainly available for patients with chronic diseases, particularly for diabetes mellitus [6,7,8,9,10,11,12,13,14].
A cellular phone is a suitable device that can be programmed for glucose monitoring without a hospital visit. In addition, it can also be used for receiving diabetes-related educational content. The majority of the previous cellular phone-based research for diabetes management used short message service (SMS) or telephone counseling. This process mainly involves telecommunication with active participation of the medical staff including medical feedback that is based on the blood glucose levels recorded by the patients. Numerous studies have already shown the multiple advantages of telecommunication for patient management and blood-glucose monitoring [15,16,17,18,19,20].
The requirements for information technology-based blood glucose monitoring are the following: a device for sensing health data, a network for communication, and medical feedback to patients. With the popularization of smart phones, many health applications have been released for these devices. However, most of the applications for chronic disease management have solely focused on self-management without interactive feedback. In contrast, clinical trials thus far have solely focused on medical feedback, thereby largely neglecting aspects pertaining to the convenience of the device or network. Consequently, in this study, we aimed to assess the effectiveness of smartphone applications for blood glucose monitoring and interactive communication between patients and the medical team.
METHODS
Study population and design
This study targeted outpatients with type 2 diabetes mellitus who visited Seoul St. Mary's Hospital from October to November 2012. For the intervention group, the inclusion criteria were men or women aged 20 to 70 years who had type 2 diabetes mellitus for more than 1 year. The baseline glycated hemoglobin (HbA1c) had to be between 7.0% and 10.0%. Patients who had serious concomitant internal diseases, such as heart failure, liver diseases, and kidney disease with a creatinine level >1.5 mg/dL or serious diabetes complications were excluded. Patients who were dependent on an insulin pump, already enrolled in other clinical trials, or otherwise considered unsuitable by the researchers were also excluded from the study.
Forty-two patients expressed their interest in participating in the study, and 38 signed the informed consent form approved by the Investigation Review Board of Seoul St. Mary Hospital. Three patients refused for reasons such as change in cell phone number. Thus, 35 patients were selected to participate in the intervention group.
At the first and the final visit, height, body-weight, waist and hip circumference, and blood pressure were measured. Blood samples were also collected for HbA1c measurement and lipid profile assessment (total cholesterol [TC], triglyceride [TG], low density lipoprotein cholesterol [LDL-C], and high density lipoprotein cholesterol [HDL-C]). The collected data were transferred to the Galaxy S-II Smartphone (Samsung Electronics, Suwon, Korea), CareSens-LINK blood glucose monitor (i-SENS, Wonju, Korea), and S(M)BPM-1 blood pressure monitor (Samsung Electronics) at the first visit. The blood glucose and blood pressure were self-measured by the patients and the recorded data were automatically transferred to the medical staff through the smartphone application (henceforth app) 'Mobile Smartcare, version 1.0.7' (Samsung Electronics). The smartphone users checked their blood glucose level and recorded the data in the smartphone application. The data were then automatically transferred to the hospital. The medical staff analyzed the data and sent recommendations and feedback tailored to the patient an average of once per week (Fig. 1). If the blood glucose level remained high, a warning message and recommendations for exercise and diet were sent to the user. If the user had hypoglycemia (<60 mg/dL) or did not record any blood glucose measurements, the medical team called the user to change the insulin dose or recommend an early visit to the hospital.
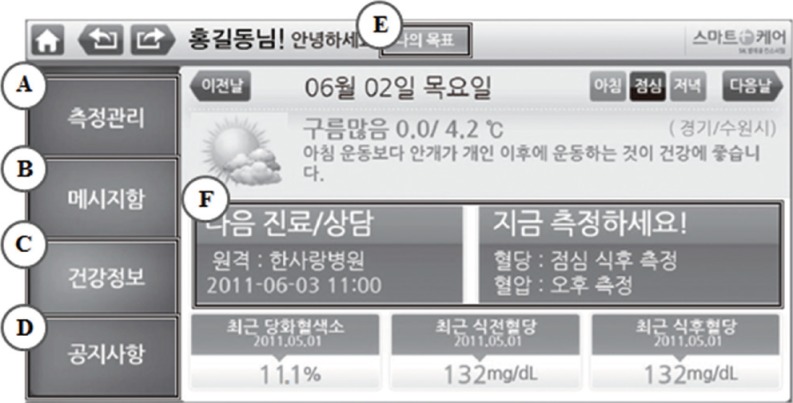
Fig. 1.
Screen view by patients on Smartphone application. (A) My laboratory data, (B) message Box from medical team, (C) health information, (D) the official announcement, (E) my target glycated hemoglobin range, blood glucose level, and blood pressure, and (F) hospital reservation guide.
After 3 months, at the second outpatient visit, the same laboratory tests and anthropometric tests were conducted to evaluate the effects of blood glucose monitoring and interactive communication. A questionnaire survey on the smartphone app was also performed at the follow-up visit. The questionnaire made by the Catholic medical team consisted of items pertaining to satisfaction, comfort, convenience, and functionality, as well as the willingness to use the app in the future and recommend to other patients.
The control group in this study was set up via electronic medical records (EMRs) review. The criteria for the control group were patients who visited the hospital with the same diagnosed disease during the same period as the intervention group. Patients matched to the intervention group in terms of age, sex, doctor in charge, and HbA1c were screened and randomly selected through EMR review. All patients were provided with the written informed consents to participate, and the study was approved by the Ethics Committee and the Review Board of Korea Institution for Social and Health Affairs.
Statistical analysis
The baseline characteristics of the two groups were compared using an unpaired t-test for continuous variables and the Mantel-Haenszel chi-square test for categorical variables. To improve the normality, we performed log-transformation for variables with a skewed distribution. We also conducted an analysis of the within-group mean change from baseline to the end of the trial using a paired t-test. Nonparametric tests were performed for subgroup analysis based on compliance. The signed-rank test was used to assess the differences in the variables between baselines and follow-up in the high and low compliance groups and the Kruskal-Wallis test was used to test differences among the three groups (high and low compliance groups, and control). Data were analyzed using a standard package (SAS version 9.1; SAS Institute, Cary, NC, USA).
RESULTS
Of the 38 patients who participated in the study, three patients dropped out due to personal reasons, and 35 patients continued until the end of the 12 week study period.
Baseline characteristics
The baseline characteristics are shown in Table 1. The mean age in the intervention group was 51.8±10.3 years, while in the control group the mean age was 53.8±9.0 years. The mean body mass index (BMI) in the intervention group was 25.0±3.3 kg/m2, and the mean duration of diabetes was 11.8±7.3 years. There were no significant differences between the two groups with respect to sex, BMI, glucose control methods and laboratory data, including HbA1c and cholesterol levels.
Table 1.
Clinical characteristics and baseline laboratory data of the Smartphone group and control groups
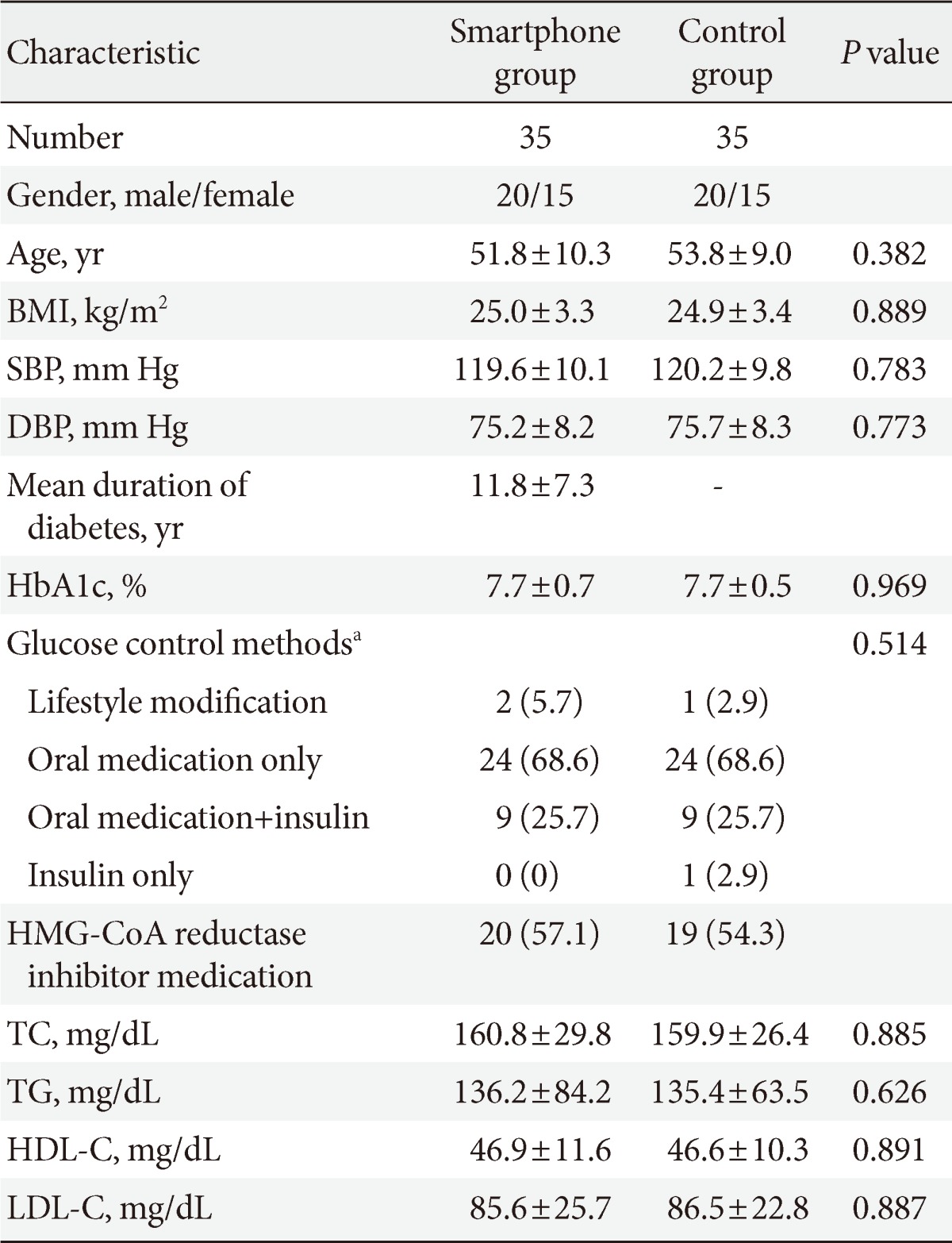
Values are presented as mean±standard deviation or number (%).
BMI, body mass index; SBP, systolic blood pressure; DBP, diastolic blood pressure; HbA1c, glycated hemoglobin; HMG-CoA, 3-hydroxy-3-methylglutaryl-CoA; TC, total cholesterol; TG, triglyceride; HDL-C, high density lipoprotein cholesterol; LDL-C, low density lipoprotein cholesterol.
aBased on Mantel-Haenszel chi-square test.
Effect on blood glucose level
In the smartphone group, the mean HbA1c decreased after 3 months from the baseline level of 7.7%±0.7% to 7.5%±0.7% (P=0.077). In the control group there was no change from baseline (7.7%±0.5% to 7.7%±0.7%) over the same period thus showing no intergroup difference after 3 months. Both the smartphone group and control group had no difference in the baseline TC, TG, HDL-C, and LDL-C levels after 3 months thus showing no significant intergroup differences (Table 2).
Table 2.
Laboratory follow-up data of subjects
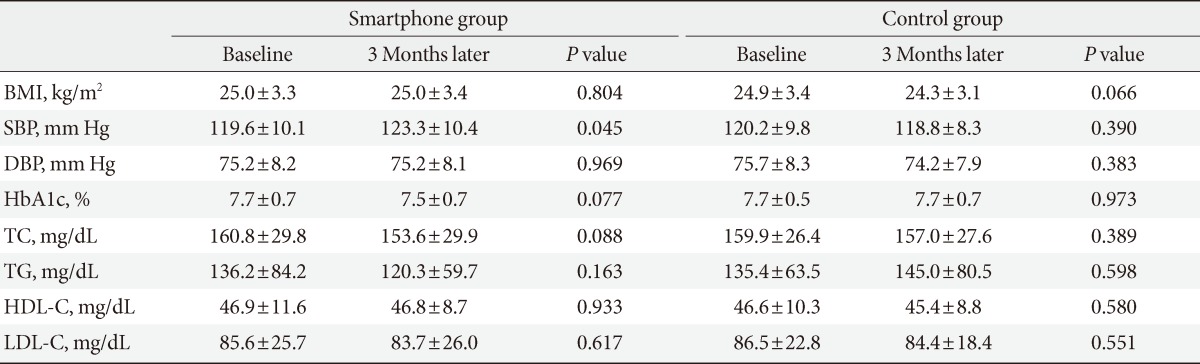
Values are presented as mean±standard deviation. Interaction between group and time about all variables were not significant.
BMI, body mass index; SBP, systolic blood pressure; DBP, diastolic blood pressure; HbA1c, glycated hemoglobin; TC, total cholesterol; TG, triglyceride; HDL-C, high density lipoprotein cholesterol; LDL-C, low density lipoprotein cholesterol.
Analysis of the questionnaire survey
Of the 35 users, two patients expressed unchanged or increased satisfaction and expectations when compared to the pre-use survey. The remaining eight patients expressed less satisfaction accompanied with complaints. Thirty-six complaints were received during the study period. The majority of these complaints were due to data transfer errors (15/36 cases, 41.7%). Other causes of complaints were mismatch between measured data and uploaded data (6/36 cases, 16.7%), network errors (5/36 cases, 13.9%), and difficulties in using the device (3/36 cases, 8.3%), which led to a decrease in satisfaction (Table 3).
Table 3.
Detailed list of complaints from the Smartphone application user
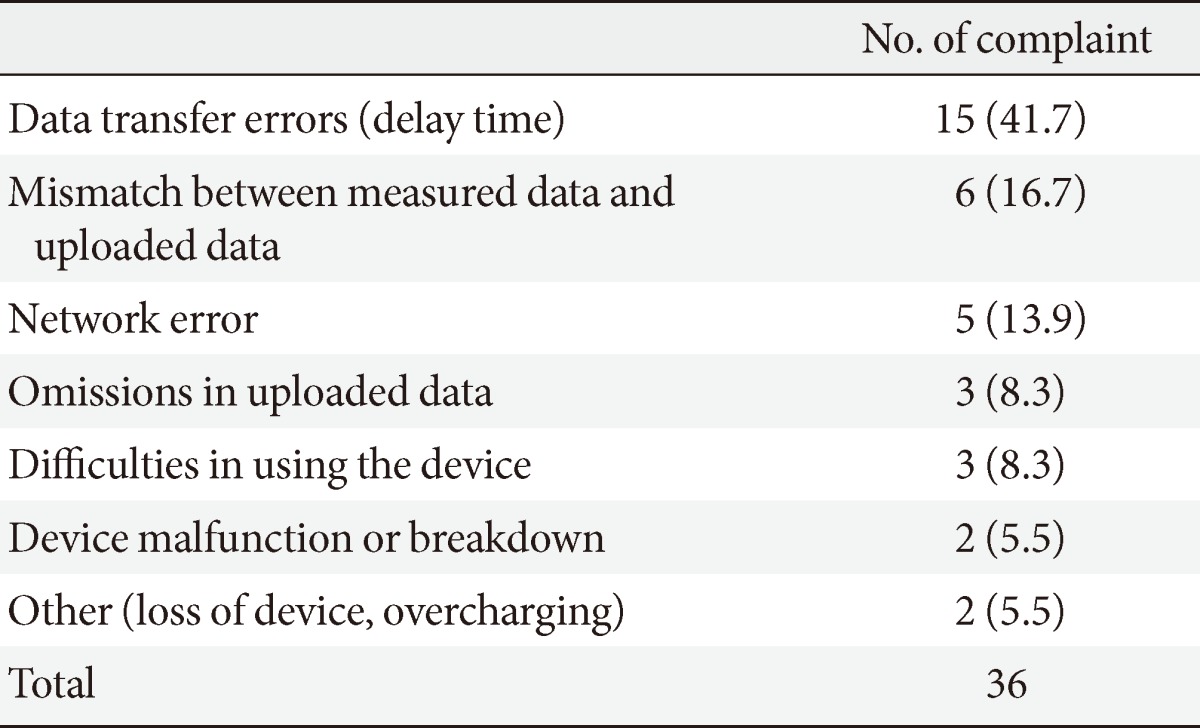
Values are presented as number (%).
Subgroup analysis by compliance
The smartphone user group was divided into two subgroups based on the results of the on the surveys conducted at baseline and after 3 months. The more satisfied group consisted of 27 patients, and the less satisfied group consisted of eight patients. There were no significant difference in age, sex, diabetes mellitus duration, blood pressure, or BMI between the more satisfied group and less satisfied group.
The change in HbA1c level from baseline to the 3-month follow-up was significantly different once satisfaction was taken into consideration (P for interaction=0.0004). The HbA1c in the more satisfied group decreased significantly from 7.7%±0.8% to 7.3%±0.6% (P<0.001), whereas in the less satisfied group, the HbA1c remained essentially the same (7.7%±0.4% to 8.1%±0.5%, P=0.062) and was actually worse than the control group. TC, TG, HDL-C, and LDL-C levels were not significantly different from the baseline levels (Table 4).
Table 4.
Laboratory follow-up data by satisfaction of patients
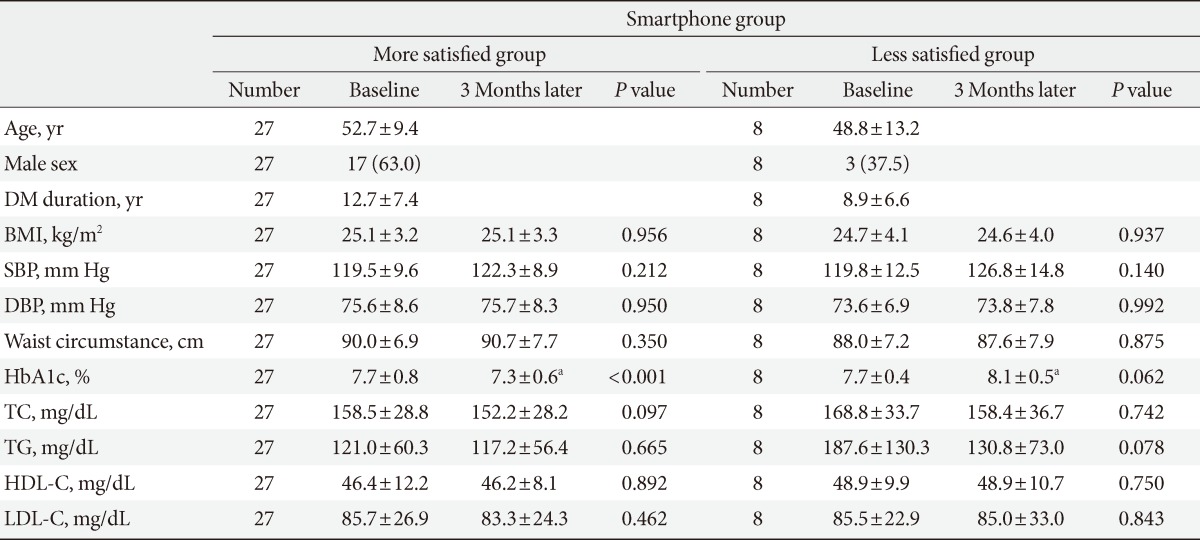
Values are presented as mean±standard deviation or number (%).
DM, diabetes mellitus; BMI, body mass index; SBP, systolic blood pressure; DBP, diastolic blood pressure; HbA1c, glycated hemoglobin; TC, total cholesterol; TG, triglyceride; HDL-C, high density lipoprotein cholesterol; LDL-C, low density lipoprotein cholesterol.
aStatistical significance was noticed (P<0.05) for the more satisfied group vs. the less satisfied group at 3 months later.
DISCUSSION
Of the three major prerequisites for IT-based health management systems, device utility (Sensors), network (Transfer), and medical feedback, existing studies have exclusively focused on medical feedback. The blood glucose monitoring system mostly revolves around active involvement of the medical staff, i.e., medical feedback. A previous study involving the use of SMS for 12 weeks demonstrated a decrease in HbA1c level by 1.01% [15], and another study on type 1 diabetes showed that the application of a cellular phone-based blood glucose monitoring system resulted in a 0.5% decrease of HbA1c levels [16]. Likewise, a number of other similar studies have shown successful reduction in the HbA1c levels [17,18,19]. These study results provide evidence regarding the feasibility of cellular phones as an important tool for blood glucose monitoring. However, it is not easy to commercialize a medical feedback-enabled smartphone, as it involves human-resource (HR) costs for the medical team that provides the medical feedback. This is directly associated with the rise of healthcare costs, thereby limiting commercialization because of low cost-effectiveness. In other words, commercialization must be preceded by economic feasibility. Several ongoing studies focus on economic analyses to investigate the possibilities of reducing medical feedback-related HR costs [19,20]. Cho et al. [20] reported a satisfactory result of a 50% reduction in medical staff by means of a simple algorithm. Methods such as a clinical decision support system and artificial intelligence that can achieve high effectiveness with low HR should be explored [20,21].
As mentioned above, the studies conducted thus far have not focused on sensor (device utility) and network transfer, which are the mediators for blood glucose monitoring. Meanwhile, this study has shown that, in addition to medical feedback, patient satisfaction with the device or network is important for blood-glucose management.
In our study, the HbA1c in the more satisfied group significantly decreased from 7.7%±0.8% to 7.3%±0.6% (P<0.001). There were no significant changes in TC, TG, HDL-C, and LDL-C. Because the study was performed over 3 months, it is unlikely that a significant change in lipid values would occur. Dissatisfaction with the use of the smartphone-based blood glucose monitoring system led to an increase in the HbA1c from 7.7%±0.4% to 8.1%±0.5% (P=0.062). The results of this study show the high positive effect on the blood glucose level in the more satisfied group after the use of smartphone compared to the less satisfied group. A notable point, however, is the negative impact on blood glucose level in the less satisfied group when compared to the no-smartphone control group. This appears to be associated with the users' dissatisfaction when an app is difficult to apply or when encountering frequent device breakdowns that affect compliance. This is immediately related to poorly controlled blood glucose. It is obvious that, in addition to medical feedback, device, and network-related patient satisfaction play a crucial role in blood glucose management. Therefore, for the smartphone app-based blood glucose monitoring to be effective, it is essential to provide the patient with a well-functioning high quality tool capable of increasing patient satisfaction and willingness to use the device. A smartphone is an upgraded type of device and network compared to the traditional cellular phone. It is simple, convenient, comfortable to carry, and appropriate for communication with the medical team. A patient who voluntarily downloads the diabetes-related app may be considered to have an interest in their blood glucose management. Nevertheless, if frequent breakdowns and other shortcomings decrease their satisfaction, then they are likely to fail in their blood glucose management. In other words, the results may be worse than if the smartphone app was not used.
Consequently, to increase user satisfaction and involve medical feedback, it is necessary to implement the steps from mobile application development to medical team involvement [22,23]. It is crucial to develop a tool that clearly reflects medical requirements and improvements. Additionally, secondary aspects, such as convenience, low cost, simple- and fun-to-use functions, and orderly design, should be considered to attract active user participation. Further, it is important to continuously upgrade the application based on user feedback. Moreover, the role of medical staff is important for the successful implementation of the fast-developing sensor and transfer app in the medical sector. It is the role of a medical team to select adequate devices, apply them, and earnestly analyze their effectiveness and economic feasibility.
This study has a few limitations. 1) First, this was not a randomized clinical trial. Nevertheless, the control group was randomly assigned by targeting patients who visited the hospital during the same period. 2) Our result is difficult to generalize because this was a small population and a short-term pilot study. 3) Therefore, additional future studies should be conducted with a larger population and longer duration. Although several blood glucose monitoring applications are available, this study used only one application; therefore, the strengths and weakness of other applications were not assessed. Given the varying functions unique to each application, the study results are likely to differ depending on the app used.
Despite these limitations, there are a few notable achievements of this study. First, a practical analysis was performed on a program attempting to combine the rapidly developing IT technology with medical needs. Second, the goal of this study was focused on the efficacy of a health management system depending on user satisfaction, which will contribute to further development of a user-friendly system. This study revealed that device utility and network can indirectly influence blood glucose management. This study may be an example of the progress in developing apps for chronic disease management in general, not only for diabetes.
The rapid development in health technology will continue, and such techniques will obviously be used more intensely in medicine. A large-scale trial investigating the possibility, efficacy, and economic feasibility is necessary to enable the effective grafting of IT to medical needs. Additionally, future efforts will have to focus on constructing a system that is capable of helping patients practically.
ACKNOWLEDGMENTS
This research was supported by the Technology Innovation Program (No. 10035059, primary care centered Smart Care Service Pilot Project) funded by the Ministry of Trade, Industry & Energy (MOTIE, Korea). The systems and devices used in this research have been provided by the SK Telecom Consortium's Smartcare Service Project. The opinions expressed in this paper are those of the authors and do not necessarily represent those of the SK Telecom Consortium's Smart Care Service Pilot Project. We thank Sun-Young Lim for collecting and inputting all of the data and analysis and Jin-Hee Lee for coordinating the study. The final version of this manuscript was approved by all authors.
Footnotes
No potential conflict of interest relevant to this article was reported.
References
- 1.Ong KL, Cheung BM, Wong LY, Wat NM, Tan KC, Lam KS. Prevalence, treatment, and control of diagnosed diabetes in the U.S. National Health and Nutrition Examination Survey 1999-2004. Ann Epidemiol. 2008;18:222–229. doi: 10.1016/j.annepidem.2007.10.007. [DOI] [PubMed] [Google Scholar]
- 2.Yoon KH, Lee JH, Kim JW, Cho JH, Choi YH, Ko SH, Zimmet P, Son HY. Epidemic obesity and type 2 diabetes in Asia. Lancet. 2006;368:1681–1688. doi: 10.1016/S0140-6736(06)69703-1. [DOI] [PubMed] [Google Scholar]
- 3.Chan JC, Malik V, Jia W, Kadowaki T, Yajnik CS, Yoon KH, Hu FB. Diabetes in Asia: epidemiology, risk factors, and pathophysiology. JAMA. 2009;301:2129–2140. doi: 10.1001/jama.2009.726. [DOI] [PubMed] [Google Scholar]
- 4.Skrøvseth SO, Arsand E, Godtliebsen F, Joakimsen RM. Model driven mobile care for patients with type 1 diabetes. Stud Health Technol Inform. 2012;180:1045–1049. [PubMed] [Google Scholar]
- 5.Lund S, Hemed M, Nielsen BB, Said A, Said K, Makungu MH, Rasch V. Mobile phones as a health communication tool to improve skilled attendance at delivery in Zanzibar: a cluster-randomised controlled trial. BJOG. 2012;119:1256–1264. doi: 10.1111/j.1471-0528.2012.03413.x. [DOI] [PubMed] [Google Scholar]
- 6.Castaldini M, Saltmarch M, Luck S, Sucher K. The development and pilot testing of a multimedia CD-ROM for diabetes education. Diabetes Educ. 1998;24:285–286. 91–92, 95–96. doi: 10.1177/014572179802400304. [DOI] [PubMed] [Google Scholar]
- 7.Frost D, Beischer W. Telemedicine in the management of pregnancy in type 1 diabetic women. Diabetes Care. 2000;23:863–864. doi: 10.2337/diacare.23.6.863. [DOI] [PubMed] [Google Scholar]
- 8.Levetan CS, Dawn KR, Robbins DC, Ratner RE. Impact of computer-generated personalized goals on HbA(1c) Diabetes Care. 2002;25:2–8. doi: 10.2337/diacare.25.1.2. [DOI] [PubMed] [Google Scholar]
- 9.McKay HG, King D, Eakin EG, Seeley JR, Glasgow RE. The diabetes network internet-based physical activity intervention: a randomized pilot study. Diabetes Care. 2001;24:1328–1334. doi: 10.2337/diacare.24.8.1328. [DOI] [PubMed] [Google Scholar]
- 10.Meigs JB, Cagliero E, Dubey A, Murphy-Sheehy P, Gildesgame C, Chueh H, Barry MJ, Singer DE, Nathan DM. A controlled trial of web-based diabetes disease management: the MGH diabetes primary care improvement project. Diabetes Care. 2003;26:750–757. doi: 10.2337/diacare.26.3.750. [DOI] [PubMed] [Google Scholar]
- 11.Meneghini LF, Albisser AM, Goldberg RB, Mintz DH. An electronic case manager for diabetes control. Diabetes Care. 1998;21:591–596. doi: 10.2337/diacare.21.4.591. [DOI] [PubMed] [Google Scholar]
- 12.Smith SA, Murphy ME, Huschka TR, Dinneen SF, Gorman CA, Zimmerman BR, Rizza RA, Naessens JM. Impact of a diabetes electronic management system on the care of patients seen in a subspecialty diabetes clinic. Diabetes Care. 1998;21:972–976. doi: 10.2337/diacare.21.6.972. [DOI] [PubMed] [Google Scholar]
- 13.Tomky DM. Developing a computerized diabetes self-management education module for documenting outcomes. Diabetes Educ. 1999;25:197–210. doi: 10.1177/014572179902500205. [DOI] [PubMed] [Google Scholar]
- 14.Yoon KH, Kim HS. A short message service by cellular phone in type 2 diabetic patients for 12 months. Diabetes Res Clin Pract. 2008;79:256–261. doi: 10.1016/j.diabres.2007.09.007. [DOI] [PubMed] [Google Scholar]
- 15.Zolfaghari M, Mousavifar SA, Pedram S, Haghani H. The impact of nurse short message services and telephone follow-ups on diabetic adherence: which one is more effective? J Clin Nurs. 2012;21:1922–1931. doi: 10.1111/j.1365-2702.2011.03951.x. [DOI] [PubMed] [Google Scholar]
- 16.Carroll AE, DiMeglio LA, Stein S, Marrero DG. Contracting and monitoring relationships for adolescents with type 1 diabetes: a pilot study. Diabetes Technol Ther. 2011;13:543–549. doi: 10.1089/dia.2010.0181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kim SI, Kim HS. Effectiveness of mobile and internet intervention in patients with obese type 2 diabetes. Int J Med Inform. 2008;77:399–404. doi: 10.1016/j.ijmedinf.2007.07.006. [DOI] [PubMed] [Google Scholar]
- 18.Istepanian RS, Zitouni K, Harry D, Moutosammy N, Sungoor A, Tang B, Earle KA. Evaluation of a mobile phone telemonitoring system for glycaemic control in patients with diabetes. J Telemed Telecare. 2009;15:125–128. doi: 10.1258/jtt.2009.003006. [DOI] [PubMed] [Google Scholar]
- 19.Shearer A, Scuffham P, Gordois A, Oglesby A. Predicted costs and outcomes from reduced vibration detection in people with diabetes in the U.S. Diabetes Care. 2003;26:2305–2310. doi: 10.2337/diacare.26.8.2305. [DOI] [PubMed] [Google Scholar]
- 20.Cho JH, Choi YH, Kim HS, Lee JH, Yoon KH. Effectiveness and safety of a glucose data-filtering system with automatic response software to reduce the physician workload in managing type 2 diabetes. J Telemed Telecare. 2011;17:257–262. doi: 10.1258/jtt.2011.101006. [DOI] [PubMed] [Google Scholar]
- 21.Ali MK, Shah S, Tandon N. Review of electronic decision-support tools for diabetes care: a viable option for low- and middle-income countries? J Diabetes Sci Technol. 2011;5:553–570. doi: 10.1177/193229681100500310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kim HS, Shin JA, Chang JS, Cho JH, Son HY, Yoon KH. Continuous glucose monitoring: current clinical use. Diabetes Metab Res Rev. 2012;28(Suppl 2):73–78. doi: 10.1002/dmrr.2346. [DOI] [PubMed] [Google Scholar]
- 23.Ko SH, Park SA, Cho JH, Ko SH, Shin KM, Lee SH, Song KH, Park YM, Ahn YB. Influence of the duration of diabetes on the outcome of a diabetes self-management education program. Diabetes Metab J. 2012;36:222–229. doi: 10.4093/dmj.2012.36.3.222. [DOI] [PMC free article] [PubMed] [Google Scholar]