Figure 2. Modified sites at C2, C3 and C4 symmetry axes in ferritin protein cages.
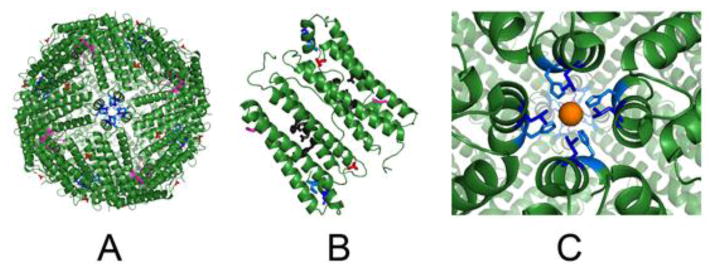
Drawn from XRD data in PDB file 3RDG, using Pymol. Ferritin protein-metal complexes were obtained by soaking apoferritin crystals in Fe2+ solutions. A. Ferritin protein cage viewed from the C4 axes (outside view); residue substitutions are shown in the 24 subunit cage. B. A pair of subunits in a 24 subunit ferritin protein cage (the subunit dimer interface is in the center). Active site residues are black sticks. C. Close up of C4 axes, in ferritin which form from the short, fifth helices, which are outside the 4-α-helix bundles and have axes almost ⊥ to the bundle axes; hydrated iron (rust) is weakly coordinating to Nε2 of H169 and in close proximity to L165. C2 loops/cage surface, E88 - red; C3 ion channels, E130 - magenta, C4 tetrameric subunit junctions of 4-helix bundles with the fifth helix, L165 - blue, H169 - blue marine.1
