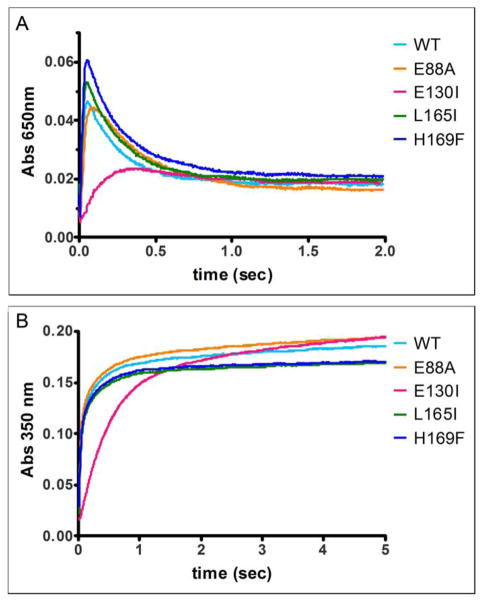
Figure 3. Selectivity for ferritin catalysis of residues at the Fe2+ entry sites (3-fold channels) and subunit dimer interfaces.
Amino acid substitution at the 4-fold ferritin cage axes had no effect on ferritin protein cage catalysis (A650 nm transient diferric peroxo enzymatic intermediate), contrasting with 2-fold (E88A) and 3-fold cage axes (E130I) axes. The absorbance maximum of A. DFP (A650 nm) and B. (Fe3+O)x species (A350 nm) were measured with rapid mixing, UV-vis spectroscopy and shown here for a set of representative curves (1 out of 3 independent analyses for each mutant). The rates (Table 1, data from 3 independent analyses) were calculated as described in the Methods. WT-wild type ferritin protein cages; E130I, ion entry channels, at 3-fold axes, E88A cage surface, near 2-fold axes, and L165I and H169F, on 4 – fold cage axes. *Significantly different than WT: P < 0.05.