Figure 5. Altered ferritin protein cages change dissolution/chelation of iron from the caged, ferritin biomineral.
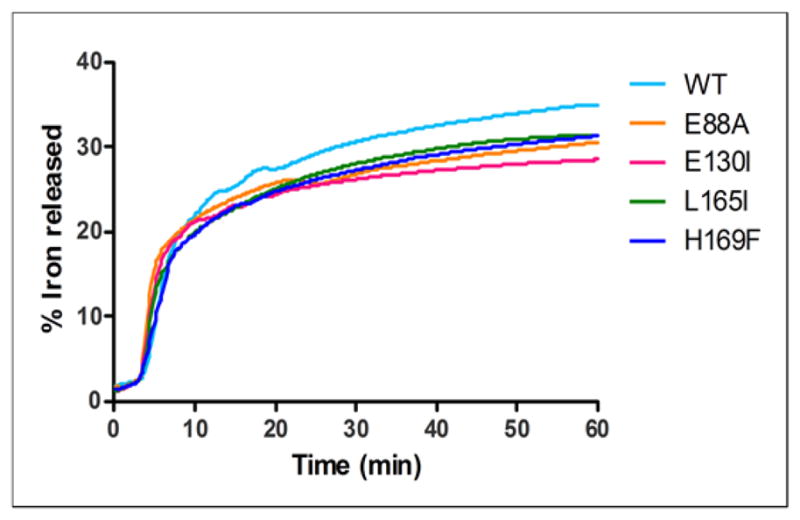
Amino acid substitution at the 4-fold cage axes (nucleation channel exits), L165I or H169F slows ferritin mineral dissolution/chelation, without effects on Fe2+ entry, contrasting with substitutions at the 3-fold axes (ion entry/exit channels), E130I, and on the surface near 2-fold axes subunit dimer interfaces (E88A), which alter both Fe2+ entry and mineral dissolution. Iron was recovered from caged minerals of hydrated ferric oxide, synthesized by and inside of ferritin protein cages, by adding reductant (NADH and FMN) in the presence of a chelator, bipyridyl, and measuring the rate for formation of [Fe (bipyridyl)3]2+ outside the protein cage as described in the Methods [6]. One representative curve for each mutant out of 5 independent experiments is reported in the figure; initial rates and % of Fe2+ released at 50 min calculated from 5 independent experiments are reported in Table 2. * Significantly different than WT: P<0.05.
